-
生物活性
Doxycycline hyclate inhibits the inflammatory response to the Lyme Disease Spirochete Borrelia burgdorferi. It is a broad spectrum inhibitor of matrix metalloproteinases in vivo.
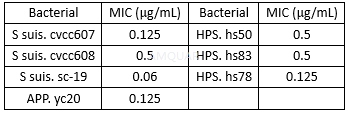
The antibacterial activity of DoxHcl (MIC: minimum inhibitory concentration)[1]
Antimalarial activity of doxycycline hyclate[2]
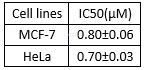
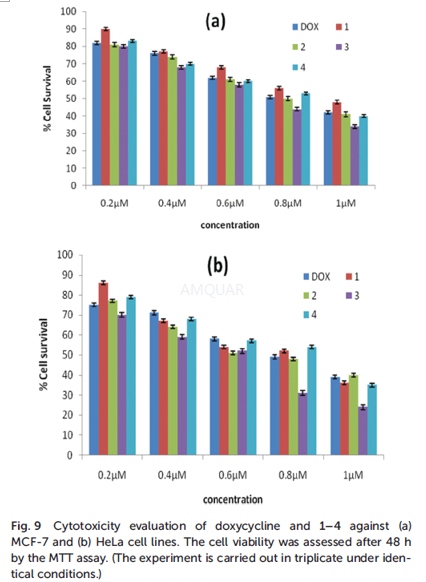
Cytotoxicity ofdoxycycline in human cervical cancer cell lines[2]
The antiviral activity in dengue NS2BNS3pro[3]
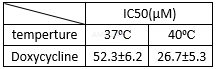
The antiviral activity of doxycycline invirusproteases[3]
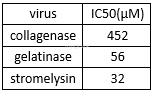
Inhibition of doxycycline for matrix metalloproteinases (MMPs) in human fibroblast[4]

Inhibition of doxycycline for MMPs or proteinase[5]
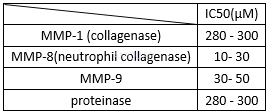
Inhibition of type I collagen degradation[6]

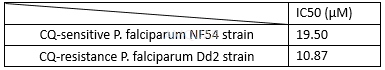
In vitro activities of doxycycline against Plasmodium falciparum[7]

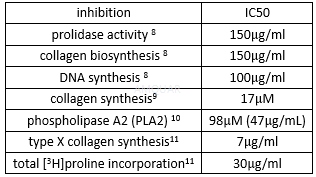
The inhibition of doxycycline
-
体外研究
-
体内研究
-
激酶实验
PLA2 enzymatic activity[10]
PLA, activity was assayed using [14C] oleic acid-labeled Escherichia coli membrane phospholipid as substrate. Reaction mixtures contained 10 mg bovine serum albumin, 2 mM CaCl2, 8 x 108radiolabeled E. coli and 0.1 M Tris-HCl buffer, pH7.5, in a volume of 1.5 mL. Reaction mixtures were incubated at 37ofor 30 min and the reaction was terminated by filtration through a 0.45pm Millipore filter. Assays were performed in duplicate in substrate excess. Enzyme activities were corrected for non-enzymatic hydrolysis.
ColIagenase assay[4]
Human interstitial collagenase (MMP-1) cDNA cloned into the EcoRI site of pUC19 encoding all but the 48 aminoterminal amino acids of full-length procollagenase, was put in frame relative to the lac promoter. The IPTG-induced collagenase was then purified from E. coli inclusion bodies by anion exchange chromatography, following solubilization in 6 M guanidine-HC1 and dialysis into 8 M urea. The enzyme was activated with stromelysin prior to assay. Rat tail3H collagen was diluted into one part 10 x Tris buffer (500mM, pH 7.5) and eight parts cold collagen ( ~ 1.2 mg/ml) and the pH adjusted to 7.5. Aliquots (50μl) were gelled overnight at 37oC in microfuge tubes. Activated collagenase (0.5μg) was added and the tubes incubated for 24 h at 37oC centrifuged at 13,000 g at 22oC for 10 min, and 50μl of the supernatants was collected and counted in 10ml Ready Safe Liquid Scintillation Cocktail for 3 min in a Beckman liquid scintillation counter.

-
细胞实验
Determination of minimum inhibitory concentration (MIC)[1]
The MIC of DoxHcl against 3 isolates of S. suis, 1 isolateof APP and 3 isolates of HPS were investigated by using a microdilution method according to the relevant standards formulatedby Clinical and Laboratory Standard Institute. Bacteria were streaked from glycerol-frozen stocks onto TSA (containing 5% fetal calf serum and 10μg/mL NAD) plates and incubated at 37oC in an atmosphere containing 5% CO2for 24 h. A single bacterial colony from the fresh plates was inoculated in the TSB (containing 5% fetal calf serum and 10μg/mL NAD) and grown at 37oC in a shaking incubator at 200 rpm to the logarithmic growth phase. The amplified bacteria were diluted to the concentration∼1 × 106cfu/mL and added to the well of a 96-well plate. The DoxHcl (100μL) was double diluted with MH broth and added to the well of the 96-well plate containing 100μL bacteria. The plate was incubated at 37oC in an atmosphere containing 5% CO2for 24 hand the MIC was determined as the lowest concentration with clear wells.
Cytotoxicity studies[2]
Cytotoxicity studies were carried out on a MCF-7 cell line. Cell viability was tested using the (3,4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay. The cells were seeded in a 24-well plate at a density of 104cells per well in DMEM containing 10% FBS and a 0.1% antibiotic solution for 24 h at 37 °C and 5% CO2for adherence. Doxycycline or other test compounds, in a concentration of 50–400μg/mL, dissolved in 1% DMSO and PBS buffer, were added to the wells with a fresh medium. 1% DMSO was used as the vehicle control. After every 24, 48, and 72 h of incubation, the MTT assay was carried out. A MTT solution (20μL, 5 mg/ mL) prepared in a 10mM phosphate buffer was added to each well and incubated for 4 h. The purple formazan product was dissolved by addition of 100μL of DMSO for 5 min. The absorbance was measured at 570 and 630 nm (for blank) using an ELISA plate reader and the viability was calculated. Data were collected for three replicates each and were used to calculatethe mean. The percentage inhibition was calculated from these data:
%inhibition = meanOD of untreated cells
(control) meanODof treated cells (control) x 100
-
动物实验
Animals[12]
Eight weeks old female homozygous (nu+/nu+) nude athymic NIH mice, 18–22 g, were used as human cancer xenograft models. The animals were housed in specific pathogen-free isolators. They were caged (six per cage) in polypropylene boxes and had free access to sterilized pelleted laboratory rodent chow and water.
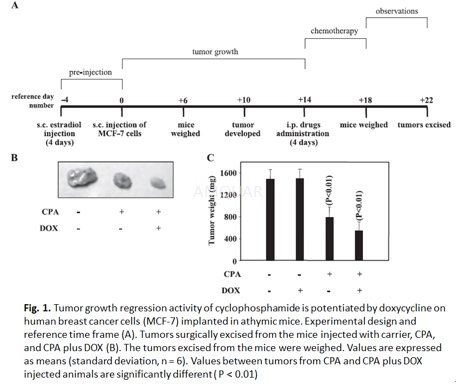
Tumor growth regression model
Five consecutive subcutaneous (sc) estradiol valerate (30μg/day) injections were administered in each mouse before sc injection of MCF-7 tumor cells in the exponential growth phase (2 x 107cells in 0.1 ml) at right flank of each mouse. Drug treatments were initiated 2 weeks after palpable tumors developed. The mice were randomized into four groups having six mice per group (n=6) to receive vehicle alone (control), doxycycline (DOX) (5mg/kg/day), cyclophosphamide (CPA) (100mg/kg/day), or CPA plus DOX by intraperitoneal (ip) injection for four consecutive days. CPA and DOX were dissolved in PBS. Four days following last injection mice were euthanized and tumors were surgically excised. Tumor growth was determined by weight measurements and host toxicity was monitored by body weight measurements.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Li X, Xie S, Pan Y, et al. Preparation, characterization and pharmacokinetics of doxycycline hydrochloride and florfenicol polyvinylpyrroliddone microparticle entrapped with hydroxypropyl-beta-cyclodextrin inclusion complexes suspension. Colloids Surf B Biointerfaces. 2016;141:634-642.
[2] Abosede OO, Vyas NA, Singh SB, et al. Copper(ii) mixed-ligand polypyridyl complexes with doxycycline - structures and biological evaluation. Dalton Trans. 2016;45(7):3003-3012.
more
分子式
C22H25ClN2O8 |
分子量
480.9 |
CAS号
10592-13-9 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
50 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02874430 | Breast Carcinoma|Endometrial Clear Cell Adenocarcinoma|Endometrial Serous Adenocarcinoma|Uterine Corpus Cancer|Uterine Corpus Carcinosarcoma | Drug: Metformin Hydrochloride|Drug: Doxycycline | Sidney Kimmel Cancer Center at Thomas Jefferson University|Thomas Jefferson University | Phase 2 | 2016-06-01 | 2016-10-18 |
| NCT03076281 | Larynx|LIP|Oral Cavity|Pharynx | Drug: Metformin Hydrochloride|Drug: Doxycycline|Drug: Metformin +Doxycycline | Sidney Kimmel Cancer Center at Thomas Jefferson University|Thomas Jefferson University | Phase 2 | 2017-12-01 | 2017-03-06 |
| NCT00803842 | Non Small Cell Lung Cancer | Drug: doxycycline | Northwestern University | | 2008-10-01 | 2015-02-24 |
| NCT00531934 | Non-Small Cell Lung Cancer | Drug: Doxycline|Drug: erlotinib [Tarceva] | Hoffmann-La Roche | Phase 2 | 2007-10-01 | 2015-02-05 |
| NCT01600625 | Meibomian Gland Dysfunction | Drug: oral minocycline hydrochloride treatment | Yonsei University | | 2011-11-01 | 2014-03-04 |
| NCT02564471 | Rabies | Drug: Chloroquine|Drug: Atovaquone and Proguanil|Drug: Doxycycline|Biological: Rabies Vaccine | State University of New York - Upstate Medical University|Walter Reed Army Institute of Research (WRAIR)|Kansas State University | Phase 4 | 2016-04-01 | 2016-04-21 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们