| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
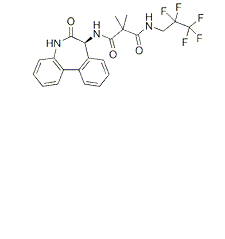
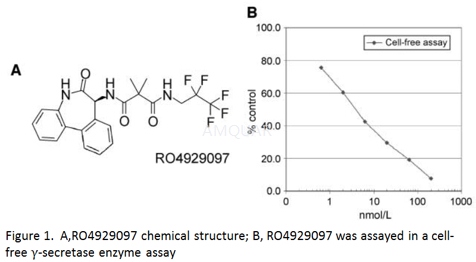
| NCT01175343 | Recurrent Fallopian Tube Carcinoma|Recurrent Ovarian Carcinoma|Recurrent Primary Peritoneal Carcinoma|Stage IV Fallopian Tube Cancer|Stage IV Ovarian Cancer|Stage IV Primary Peritoneal Cancer | Drug: Gamma-Secretase Inhibitor RO4929097|Other: Laboratory Biomarker Analysis | National Cancer Institute (NCI) | Phase 2 | 2010-07-01 | 2016-10-21 |
| NCT01141569 | Clear Cell Renal Cell Carcinoma|Recurrent Renal Cell Carcinoma|Stage IV Renal Cell Cancer | Drug: Gamma-Secretase Inhibitor RO4929097|Other: Laboratory Biomarker Analysis | National Cancer Institute (NCI) | Phase 2 | 2010-06-01 | 2017-01-19 |
| NCT01198535 | Colon Mucinous Adenocarcinoma|Colon Signet Ring Cell Adenocarcinoma|Rectal Mucinous Adenocarcinoma|Rectal Signet Ring Cell Adenocarcinoma|Recurrent Colon Carcinoma|Recurrent Rectal Carcinoma|Stage IVA Colon Cancer|Stage IVA Rectal Cancer|Stage IVB Colon Cancer|Stage IVB Rectal Cancer | Biological: Cetuximab|Drug: Gamma-Secretase Inhibitor RO4929097|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study | National Cancer Institute (NCI) | Phase 1 | 2010-09-01 | 2015-05-15 |
| NCT01232829 | Adenocarcinoma of the Pancreas|Recurrent Pancreatic Cancer|Stage IV Pancreatic Cancer | Drug: gamma-secretase/Notch signalling pathway inhibitor RO4929097 | National Cancer Institute (NCI) | Phase 2 | 2010-10-01 | 2014-11-19 |
| NCT01238133 | Estrogen Receptor Negative|HER2/Neu Negative|Progesterone Receptor Negative|Stage IIA Breast Cancer|Stage IIB Breast Cancer|Stage IIIA Breast Cancer|Stage IIIB Breast Cancer|Stage IIIC Breast Cancer|Triple-Negative Breast Carcinoma | Drug: Carboplatin|Drug: Gamma-Secretase Inhibitor RO4929097|Other: Laboratory Biomarker Analysis|Drug: Paclitaxel|Other: Pharmacological Study|Procedure: Therapeutic Conventional Surgery | National Cancer Institute (NCI) | Phase 1 | 2010-12-01 | 2015-09-03 |
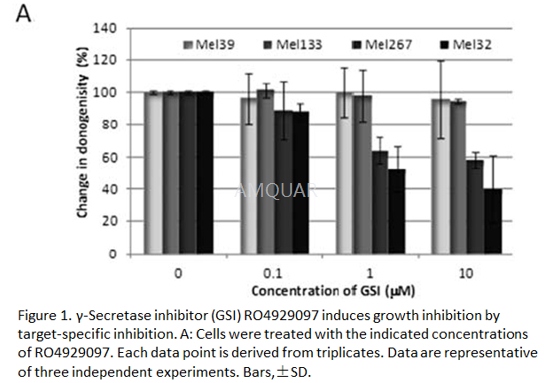
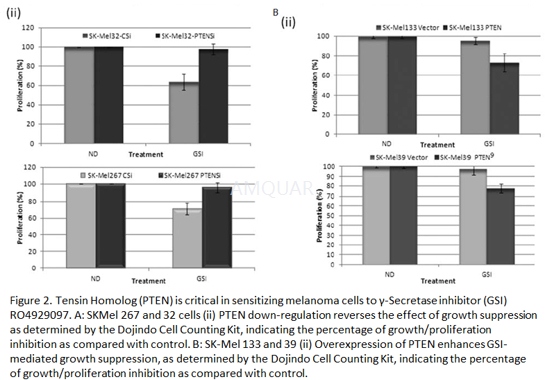
| NCT01120275 | Acral Lentiginous Malignant Melanoma|Lentigo Maligna Malignant Melanoma|Nodular Malignant Melanoma|Recurrent Melanoma|Solar Radiation-related Skin Melanoma|Stage IV Melanoma|Superficial Spreading Malignant Melanoma | Drug: gamma-secretase/Notch signalling pathway inhibitor RO4929097|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 2010-10-01 | 2016-05-06 |
| NCT01122901 | Adult Giant Cell Glioblastoma|Adult Glioblastoma|Adult Gliosarcoma|Recurrent Adult Brain Tumor | Drug: gamma-secretase/Notch signalling pathway inhibitor RO4929097|Procedure: therapeutic conventional surgery|Other: pharmacological study|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 2010-12-01 | 2016-09-20 |
| NCT01193881 | Recurrent Non-Small Cell Lung Carcinoma|Stage IV Non-Small Cell Lung Cancer | Drug: Erlotinib Hydrochloride|Drug: Gamma-Secretase Inhibitor RO4929097|Other: Laboratory Biomarker Analysis|Other: Pharmacological Study | National Cancer Institute (NCI) | Phase 1 | 2010-08-01 | 2015-09-28 |
| NCT01116687 | Recurrent Colon Cancer|Recurrent Rectal Cancer|Stage IV Colon Cancer|Stage IV Rectal Cancer | Drug: gamma-secretase/Notch signalling pathway inhibitor RO4929097|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 2010-05-01 | 2014-05-02 |
| NCT01193868 | Recurrent Non-small Cell Lung Cancer|Stage IIIB Non-small Cell Lung Cancer|Stage IV Non-small Cell Lung Cancer | Drug: gamma-secretase/Notch signalling pathway inhibitor RO4929097|Other: laboratory biomarker analysis|Other: pharmacological study | National Cancer Institute (NCI)|M.D. Anderson Cancer Center | Phase 2 | 2010-09-01 | 2015-10-15 |
| NCT01216787 | Stage IIIB Melanoma|Stage IIIC Melanoma|Stage IV Melanoma | Drug: gamma-secretase/Notch signalling pathway inhibitor RO4929097|Other: pharmacological study|Procedure: therapeutic conventional surgery|Other: laboratory biomarker analysis | National Cancer Institute (NCI) | Phase 2 | 2010-09-01 | 2013-01-30 |
| NCT01251172 | DS Stage I Plasma Cell Myeloma|DS Stage II Plasma Cell Myeloma|DS Stage III Plasma Cell Myeloma|Refractory Plasma Cell Myeloma | Procedure: Autologous Hematopoietic Stem Cell Transplantation|Drug: Gamma-Secretase Inhibitor RO4929097|Procedure: Hematopoietic Stem Cell Mobilization|Procedure: In Vitro-Treated Peripheral Blood Stem Cell Transplantation|Other: Laboratory Biomarker Analysis|Drug: Melphalan | National Cancer Institute (NCI) | Phase 2 | 2010-12-01 | 2017-02-27 |
| NCT01433406 | Diabetes Mellitus | Drug: TAK-875|Drug: TAK-875 | Takeda | Phase 3 | 2011-10-01 | 2014-01-24 |
| NCT01414920 | Diabetes Mellitus, Type 2 | Drug: Placebo|Drug: TAK-875|Drug: TAK-875|Drug: Sitagliptin|Drug: TAK-875 and Sitagliptin|Drug: TAK-875 and Sitagliptin | Takeda | Phase 2 | 2011-08-01 | 2016-03-04 |
| NCT01007097 | Diabetes Mellitus, Type 2 | Drug: TAK-875|Drug: TAK-875|Drug: TAK-875|Drug: TAK-875|Drug: TAK-875|Drug: Glimepiride|Drug: Placebo | Takeda | Phase 2 | 2009-12-01 | 2016-03-13 |
| NCT01433393 | Diabetes Mellitus | Drug: TAK-875|Drug: TAK-875|Drug: Placebo | Takeda | Phase 3 | null | 2012-11-07 |
| NCT01585792 | Diabetic Patients | Drug: TAK-875|Drug: TAK-875|Drug: Glimepiride|Drug: Placebo | Takeda | Phase 3 | 2012-05-01 | 2013-04-15 |