-
生物活性
Entospletinib is a highly selective and orally efficacious Syk inhibitor(IC50= 7.7 nM) that is currently undergoing clinical evaluation for autoimmune and oncology indications.
Activity of Sky[1]

GS-9973 shows only modest activity in Gebhard Thoma’s assays[5]

The ability of GS-9973 to inhibit CYP1A2, 2C9, 2C19, 2D6, and 3A4: IC50 values were more than 10 μM in all cases.[1]
-
体外研究
-
体内研究
-
激酶实验
Syk Kinase Assay[5]
The assay was performed as end-point determination using 384-well microtiter plates. Compounds were tested as 8-point dose responses. The assays were prepared by addition of 50nL of compound solution in 90% DMSO directly into the empty plate using a hummingbird dispenser. Subsequently, 4.5 μL of a mixture of 4 μM ATP and 4 μM peptide (5- Fluo-Ahx-GAPDYENLQELNKK-Amide) in reaction buffer (50 mM HEPES, pH 7.5, 1 mM DTT, 0.02% Tween-20, 0.02% BSA, 0.6% DMSO, 10 mM beta-glycerophosphate, 10 μM sodium orthovanadate, 1 mM MgCl2, 3 mM MnCl2) was added to each well. The kinase reactions were started by further addition of 4.5 μL of enzyme solution (4 nM Syk (2-635) in reaction buffer). After 60 min incubation at 30°C, reactions were terminated by addition of 16 μL per well of EDTA stop solution. Product formation was measured in a microfluidic mobility shift assay.
-
细胞实验
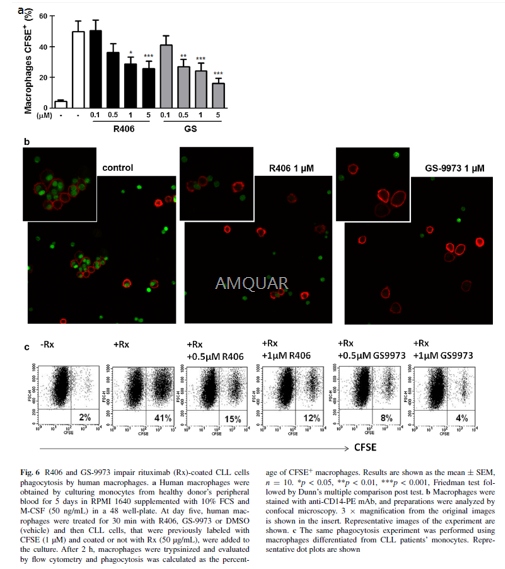
Phagocytosis assay[3]
Macrophages were differentiated from healthy donors’ or chronic lymphocytic leukemia patients’ monocytes by culturing them for 5 days in RPMI 1640 with 10% FCS and M-CSF (50 ng/mL). For the phagocytosis assay, macrophages were treated for 30 min with R406, GS-9973 or DMSO and then CLL cells, that were previously labeled with CFSE (1 μM) and coated or not with rituximab (50 μg/mL), were added to the culture. After 2 h, macrophages were trypsinized and the phagocytosis was evaluated by flow cytometry. Macrophages were determined by morphology in the FSC-H and SSC-H dot plot. The expression of CD20 was evaluated in CLL cells by flow cytometry after 48 h of treatment with DMSO, R406 or GS-9973. Phagocytosis was also evaluated by confocal microscopy. To this aim, after the phagocytosis assay, macrophages were trypsinized, stained with anti-CD14-PE mAb and then centrifuged onto cytospin slides and coverslips were mounted using Fluoromount-G. Immunofluorescence images were acquired with a FluoView FV1000 confocal microscope using a Plapon 60 × 1.42 NA oil immersion objective, and images were analyzed using the Olympus FV10-ASW software.
-
动物实验
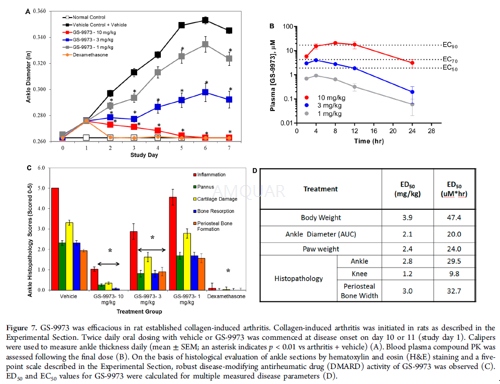
Rat Collagen-Induced Arthritis (CIA) Model[1]
Female Lewis rats (mean mass 178 g, eight per group for collagen arthritis, four per group for normal controls) were anesthetized with isoflurane and injected with 300μL of Freund’s incomplete adjuvant containing 2 mg/mL bovine type II collagen at the base of the tail and two sites on the back on days 0 and 6. Oral dosing (bid at 12 h intervals) was performed on arthritic days 0−7 with vehicle (Cremophor/ethanol/saline), GS-9973 (1, 3, or 10 mg/kg), or the reference compound dexamethasone (Dex; 0.075 mg/kg) administered daily (qd). Rats were terminated on arthritis day 16. Efficacy evaluation was based on animal body masses, daily ankle caliper measurements, ankle diameters expressed as the area under the curve (AUC), terminal hind paw masses, and histopathologic evaluation of ankles and knees. PK was measured from plasma samples taken 0, 2, 4, 8, 12, and 24 h post last dose. The paws were fixed in formalin and processed for hemotoxylin (H) and eosin (E) microscopy. H and E sections were scored for bone resorption as follows: (0) normal; (0.5) normal on low magnification but have the earliest hint of small areas of resorption in the metaphysis with no resorption in the tarsal bones; (1) (minimal) small definite areas of resorption in distal tibial trabecular or cortical bone or in the tarsal bones, not readily apparent on low magnification, rare osteoclasts; (2) (mild) more numerous areas (<25% loss of bone in growth plate area) of resorption in distal tibial trabecular or cortical bone and tarsals apparent on low magnification, osteoclasts more numerous; (3) (moderate) obvious resorption of medullary trabecular and cortical bone without full thickness defects in both distal tibial cortices, loss of some medullary trabeculae with 26−50% loss across the growth plate and cortices, some loss in tarsal bones, lesion apparent on low magnification, osteoclasts more numerous; (4) (marked) full or nearly full thickness defects in both distal tibial cortices, often with distortion of the profile of the remaining cortical surface, marked loss of medullary bone of distal tibia (50−100% loss across the growth plate area and cortices and up to 50% loss in small tarsals if minor in tibia), numerous osteoclasts, minor to mild resorption in smaller tarsal bones; (5) (severe) full thickness defects in both distal tibial cortices with >75% loss across the growth plate and both cortices and >50% loss in tarsals, often with distortion of the profile of the remaining cortical surface, marked loss of medullary bone of distal tibia, numerous osteoclasts. Osteoclast counts (5400× fields) were performed on the ankles in the areas of greatest bone resorption.
For statistical analysis, the ankle thicknesses, bone erosion scores, osteoclast counts, and c-fos expression values (mean ± SE) were analyzed for group differences using the Student’s t test. Significance was set at p < 0.05.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Currie KS, Kropf JE, Lee T, et al. Discovery of GS-9973, a selective and orally efficacious inhibitor of spleen tyrosine kinase. J Med Chem. 2014;57(9):3856-3873.
[2] Chapuy B, Cheng H, Watahiki A, et al. Diffuse large B-cell lymphoma patient-derived xenograft models capture the molecular and biological heterogeneity of the disease. Blood. 2016;127(18):2203-2213.
more
分子式
C23H21N7O |
分子量
411.46 |
CAS号
1229208-44-9 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
86 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02521376 | Oncology | Drug: Entospletinib | Gilead Sciences | Phase 1 | 2015-11-01 | 2017-02-10 |
| NCT01796470 | Chronic Lymphocytic Leukemia|Mantle Cell Lymphoma|Diffuse Large B-cell Lymphoma|Indolent Non-Hodgkin's Lymphoma | Drug: Entospletinib|Drug: Idelalisib | Gilead Sciences | Phase 2 | 2013-06-01 | 2017-01-20 |
| NCT01799889 | Chronic Lymphocytic Leukemia|Mantle Cell Lymphoma|Diffuse Large B-cell Lymphoma|Non-FL Indolent Non-Hodgkin's Lymphoma|Follicular Lymphoma | Drug: Entospletinib|Drug: Entospletinib SDD | Gilead Sciences | Phase 2 | 2013-03-14 | 2017-02-27 |
| NCT02404220 | Acute Lymphoid Leukemia | Drug: Entospletinib|Drug: VCR|Drug: Dexamethasone|Drug: CNS Prophylaxis | Gilead Sciences | Phase 1 | 2015-05-28 | 2017-02-23 |
| NCT02701634 | Chronic Graft Versus Host Disease | Drug: Entospletinib|Drug: Placebo|Drug: Systemic Corticosteroids | Gilead Sciences | Phase 2 | 2016-05-27 | 2017-02-27 |
| NCT02343939 | Acute Myeloid Leukemia | Drug: Entospletinib|Drug: Daunorubicin|Drug: Cytarabine|Drug: Decitabine|Drug: ARA-C|Drug: Azacitidine|Drug: MEC | Gilead Sciences | Phase 1|Phase 2 | 2015-07-01 | 2017-02-16 |
| NCT02983617 | Chronic Lymphocytic Leukemia | Drug: GS-4059|Drug: Entospletinib|Drug: Obinutuzumab | Gilead Sciences|German CLL Study Group | Phase 2 | 2017-02-01 | 2017-02-13 |
| NCT03010358 | Anemia|B-Cell Prolymphocytic Leukemia|Fatigue|Fever|Grade 1 Follicular Lymphoma|Grade 2 Follicular Lymphoma|Grade 3a Follicular Lymphoma|Hairy Cell Leukemia|Lymphadenopathy|Lymphocytosis|Lymphoplasmacytic Lymphoma|Mantle Cell Lymphoma|Marginal Zone Lymphoma|Night Sweats|Recurrent Chronic Lymphocytic Leukemia|Recurrent Small Lymphocytic Lymphoma|Refractory Chronic Lymphocytic Leukemia|Refractory Small Lymphocytic Lymphoma|Richter Syndrome|Splenomegaly|Thrombocytopenia|Weight Loss | Drug: Entospletinib|Other: Laboratory Biomarker Analysis|Biological: Obinutuzumab|Other: Pharmacological Study | OHSU Knight Cancer Institute|National Cancer Institute (NCI) | Phase 1|Phase 2 | 2017-03-01 | 2017-02-16 |
| NCT02568683 | Non-Hodgkin Lymphoma | Drug: ENTO|Drug: VCR | Gilead Sciences | Phase 1|Phase 2 | 2016-02-01 | 2017-01-13 |
| NCT01841489 | Chronic Lymphocytic Leukemia | Drug: Treatment A|Drug: Treatment B|Drug: Treatment C|Drug: Treatment D|Drug: Treatment E|Drug: Treatment F|Drug: Treatment G|Drug: Treatment H|Drug: Treatment I|Drug: Treatment J | Gilead Sciences | Phase 1 | 2013-05-01 | 2014-02-05 |
| NCT02457598 | B-cell Malignancies | Drug: ONO/GS-4059|Drug: Idelalisib|Drug: Entospletinib|Drug: Obinutuzumab | Gilead Sciences | Phase 1 | 2015-06-30 | 2017-01-26 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们





















