-
生物活性
PARP1 inhibitor. Inhibits growth of certain breast cancer cell lines in vitro. Non-selectively modifies cysteine-containing proteins in tumor cells. Inhibits ionizing radiation-induced DNA single-stranded breaks in lymphoid cell lines in vivo.
The IC50 values in the PARP-1/2enzymatic assays and EC50 values in the PARP cellular assays[1]

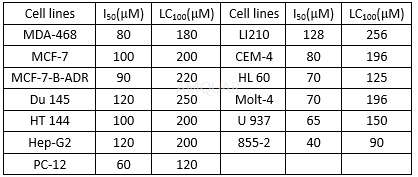
Cytotoxicity of INO2BA in various cancer cell lines[2]
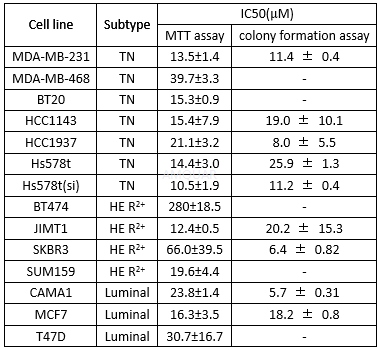
Effect in breast cancer cell lines[3]
-
体外研究
-
体内研究
-
激酶实验
PARP enzymatic assay[1]
PARP enzymatic assays were conducted in buffer containing 50 mmol/L Tris pH 8.0, 1 mmol/L dithiothreitol (DTT), 1.5 mmol/L [3H]-NAD+(1.6 mCi/mmol), 200 nmol/L biotinylated histone H1, 200 nmol/L DNA oligo (CACAAGTGTTGCATTCCTCTCTGAAGTTAAGACCTATGCAGAGAGGAATGCAA- CACTTGTG), and 1 nmol/L PARP-1 or 4 nmol/L PARP-2 enzyme. Compounds were assayed as 11-point, 3-fold dilution series from 10μmol/L to 170 pmol/L in 2% dimethyl sulfoxide (DMSO). Reactions were carried out in 100-μL volumes in white 96-well plates. These reactions were terminated after 1 hour by the addition of 150μL of 1.5 mmol/L benzamide (~1,000-fold more than its IC50). Aliquots of 170 mL of the stopped reaction mixtures were transferred to streptavidin Flash Plates, incubated for 16 hours, and counted using a TopCount microplate scintillation counter.
-
细胞实验
Cell Culture[4]
Mouse embryo fibroblasts (MEFs) from wildtype or Atm−/−mice were cultured in DMEM (medium A). GM16666 and GM16667 human fibroblast lines were cultured in DMEM with 100μg/ml hygromycin. PEO1 and PEO4 cells were cultured in DMEM containing 100 μM nonessential amino acids and 10 μg/ml insulin. SKOV3 cells were cultured in McCoy’s 5A. All media contained 10% heat inactivated fetal bovine serum, 40 units/ml penicillin G, 40 μg/ml streptomycin, and 1 mM glutamine. Lines were genotyped shortly before acquisition and were reinitiated every 2–3 months from stocks that were cryopreserved immediately after receipt from the indicated sources.
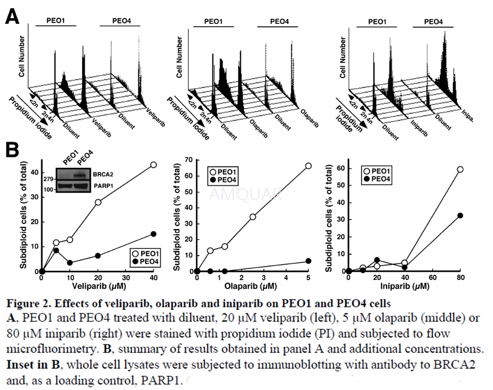
ApoptosisAssays
Cells plated at 5 × 104cells/60 mm dish were allowed to adhere for 24 h, and then treated with veliparib, olaparib, or iniparib in 0.1% (v/v) DMSO for 4 days (olaparib) or 6 days (veliparib, iniparib) based on preliminary time course experiments. At the end of treatment, adherent cells were recovered by trypsinization, combined with cells in the supernatant, sedimented at 150 × g for 10 min, washed in ice-cold calcium- and magnesium-free Dulbecco’s phosphate-buffered saline (PBS), and fixed at 4° C by drop-wise addition of ethanol to a final concentration of 50% (v/v). Cells were subsequently rehydrated in cold PBS, sedimented, resuspended in 300 μl 0.1% (w/v) sodium citrate containing 1 mg/ml RNase A, incubated for 15 min at 37 °C, diluted with 300μl 0.1% sodium citrate containing 100 μg/ml PI, incubated in the dark at 20 °C for 15 min, and analyzed (20,000 events) on a FACSCanto II flow cytometer using excitation and emission wavelengths of 488 and 617 nm, respectively. After data were analyzed using Becton Dickinson CellQuest software, normalized apoptosis was calculated as (observed –baseline)/(100 – baseline) to correct for differences in basal apoptosis rates between cell lines. Results are representative of at least 3 independent experiments.
-
动物实验
Capan-1 xenograft tumor study[1]
For this study, C.B.-17 severe combined immunodeficient mice (SCID) female mice were obtained from Charles River. Mouse body weight at initiation of therapy was~21 g. Capan-1 brei was prepared by homogenizing 30 grams of tumor tissue with 30 mL media. Matrigel was added to the mixture (1:1) to complete the brei. An inoculation volume of 0.2 mL was injected into the right hind flank of the C.B.-17 SCID female mice on day 0. Singleagent therapy was administered per os, twice a day x14 days for veliparib and intraperitoneal (i.p.), twice a day x14 days for iniparib starting from day 25. Tumor volume was calculated twice weekly. Measurements of the length (L) and width (W) of the tumor were taken via electronic caliper and the volume was calculated according to the following equation: V=LxW2/2 using Study Director Version 1.7.39. The percent tumor growth inhibition (% TGI) was calculated using the formula 100-%T/C (drug-treated/vehicle-treated tumor volumex100). Data were analyzed using the Student t test for differences in T/C values.
B16F10 xenograft tumor study
C57BL/6 female mice were obtained from Charles River. Mouse body weight at initiation of therapy was ~22 g. A total of 6 x104viable B16F10 cells were inoculated subcutaneously into the right flank of female C57BL/6 mice on day 0. The injection volume was 0.1mLand comprised a 1:1 mixture of GIBCO Minimum Essential Medium (S-MEM) and Matrigel. Veliparib was administered per os twice a day x 5 days and iniparib was administered i.p. twice a day x 5 days starting from day 7. Temozolomide was administered per os every day x 5 days. Tumor volume was calculated 3 times weekly. Tumor volume calculation, % TGI calculation, and data analysis are the same as in the Capan-1 xenograft tumor study.
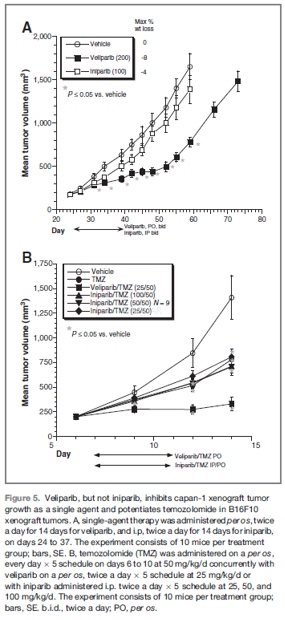
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Liu X, Shi Y, Maag DX, et al. Iniparib nonselectively modifies cysteine-containing proteins in tumor cells and is not a bona fide PARP inhibitor. Clin Cancer Res. 2012;18(2):510-523.
[2] Mendeleyev J KE, Hakam A, Buki KG, Kun E. Potential chemotherapeutic activity of 4-iodo-3-nitrobenzamide. Metabolic reduction to the 3-nitroso derivative and induction of cell death in tumor cells in culture. Biochem Pharmacol. 1995;50(5):705-714.
more
分子式
C7H5IN2O3 |
分子量
292.03 |
CAS号
160003-66-7 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
≥56.4 mg/mL |
Water
<1 mg/mL |
Ethanol
30 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01161836 | Advanced Solid Tumors | Drug: Iniparib | Sanofi | Phase 1 | 2010-07-01 | 2013-09-23 |
| NCT00677079 | Primary Peritoneal Cancer|Advanced Epithelial Ovarian Cancer | Drug: Iniparib | Sanofi | Phase 2 | 2008-06-01 | 2013-09-23 |
| NCT01173497 | Estrogen Receptor Negative (ER-Negative) Breast Cancer|Progesterone Receptor Negative (PR-Negative) Breast Cancer|Human Epidermal Growth Factor Receptor 2 Negative (HER2-Negative) Breast Cancer|Brain Metastases | Drug: INIPARIB + irinotecan | Sanofi|UNC Lineberger Comprehensive Cancer Center | Phase 2 | 2010-07-01 | 2016-02-17 |
| NCT01130259 | Breast Cancer | Drug: iniparib | Sanofi | | null | 2013-09-14 |
| NCT01551680 | Brain Metastases | Radiation: Radiation combined with iniparib (BSI-201) | Institut du Cancer de Montpellier - Val d'Aurelle | Phase 1 | 2012-09-01 | 2015-03-05 |
| NCT01033292 | Ovarian Cancer | Drug: BSI-201 | Sanofi | Phase 2 | 2009-12-01 | 2016-02-17 |
| NCT01033123 | Ovarian Cancer | Drug: BSI-201 | Sanofi | Phase 2 | 2009-12-01 | 2016-02-17 |
| NCT00298675 | Tumors | Drug: BSI-201 (iniparib)|Drug: irinotecan | Sanofi | Phase 1 | 2006-03-01 | 2012-08-01 |
| NCT01045304 | Breast Cancer, Metastatic | Drug: Iniparib|Drug: Gemcitabine|Drug: Carboplatin | Sanofi | Phase 2 | 2010-02-01 | 2014-01-13 |
| NCT01455532 | Neoplasm Malignant | Drug: Iniparib (SAR240550-BSI-201)|Drug: Gemcitabine|Drug: Carboplatin|Drug: Placlitaxel|Drug: Pegylated liposomal doxorubicin | Sanofi | Phase 1 | 2011-11-01 | 2014-10-21 |
| NCT01086254 | Non-small Cell Lung Cancer Stage IV | Drug: Iniparib|Drug: gemcitabine|Drug: cisplatin | Sanofi | Phase 2 | 2010-05-01 | 2013-09-23 |
| NCT00540358 | Breast Cancer | Drug: gemcitabine/carboplatin|Drug: iniparib | Sanofi|BiPar Sciences | Phase 2 | 2007-10-01 | 2012-12-21 |
| NCT00938652 | Breast Cancer | Drug: gemcitabine/carboplatin|Drug: Iniparib | Sanofi | Phase 3 | 2009-07-01 | 2013-09-13 |
| NCT01082549 | Squamous Cell Lung Cancer | Drug: gemcitabine/carboplatin|Drug: gemcitabine/carboplatin plus Iniparib | Sanofi | Phase 3 | 2010-03-01 | 2016-02-17 |
| NCT00687687 | Uterine Carcinosarcoma | Drug: paclitaxel|Drug: carboplatin|Drug: BSI-201 (Iniparib) | Sanofi|Gynecologic Oncology Group | Phase 2 | 2008-05-01 | 2012-08-01 |
| NCT00687765 | Glioblastoma | Drug: bsi-201 plus temozolomide | Sanofi | Phase 1|Phase 2 | 2008-07-01 | 2015-07-10 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们