-
生物活性
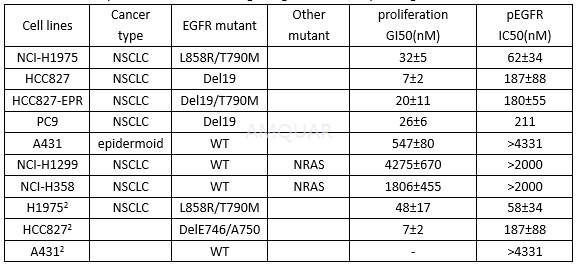
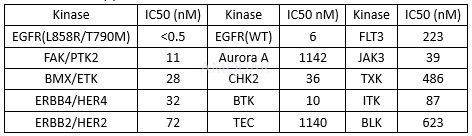
Rociletinib is a potent inhibitor of EGFR L858R/T790M kinase (kinact/Ki = (2.41 ± 0.30) x 105 M-1s-1) and is approximately 22-fold selective over WT EGFR (kinactt/Ki = (1.12 ± 0.14) x 104 M-1s-1). Rociletinib potently inhibited proliferation in the mutant-EGFR NSCLC cellswith GI50 values ranging from 7 - 32 nM. In comparison, the GI50 value for A431 cells, an epidermoid cell line that is driven by amplified WT EGFR(19), was 547 nM.
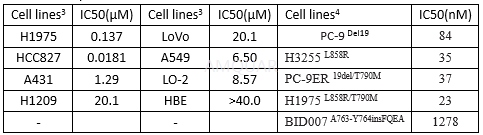
Sensitivity of CO-1686–resistant NCI-1975 cell clones to EGFR TKIs[1]

Inhibition of cell proliferation and EGFR signaling in cell lines expressing mutant EGFR[1][2]
Kinase selectivity profile at 1 mmol/L[2]
Cellular antiproliferative activities
-
体外研究
-
体内研究
1% DMSO+30% polyethylene glycol+1% Tween 80
-
激酶实验
Kinase Enzymatic Assays[3]
The wild-type EGFR kinase enzyme system and the T790M/L858R-mutated EGFR kinase enzyme were purchased. Concentrations consisting of suitable levels from 0.1 to 100 nM were used for all of the tested compounds. The experiments were performed according to the instructions of the manufacturer. The test was performed in a 384-well plate, and includes the major steps below:
(1) perform a 5μl kinase reaction using 1x kinase buffer (e.g., 1x reaction buffer A); (2) incubate at room temperature for 60 min; (3) add 5μl of ADP-Glo™ Reagent to stop the kinase reaction and deplete the unconsumed ATP, leaving only ADP and a very low background of ATP; (4) incubate at room temperature for 40 min; (5) add 10μl of Kinase Detection; (6) reagent to convert ADP to ATP and introduce luciferase and luciferin to detect ATP; (7) incubate at room temperature for 30 min; (8) plate was measured on TriStar® LB942 Multimode Microplate Reader to detect the luminescence (Integration time 0.5–1 s). Curve fitting and data presentations were performed using GraphPad Prism version 5.0.
-
细胞实验
Cell Culture[1]
NCI-H1975, HCC827, HCC-H1299, NCIH358, and PC-9 cells were grown in RPMI-1640 supplemented with 10% FBS, 2mmol/L L-glutamine, and 1% penicillin–streptomycin. A431 cells were grown in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10% FBS, 2mmol/L L-glutamine, and 1% penicillin–streptomycin. HCC827-EPR cells were grown in RPMI-1640 supplemented with 10% FBS, 2mmol/L L-glutamine, 1% penicillin–streptomycin, 1 μmol/L erlotinib, and 1μmol/L PHA-665752. PC-9/ER and H3255/XLR cells were grown in the same media as above supplemented with 1μmol/L erlotinib. All cells were maintained and propagated as monolayer cultures at 37°C in a humidified 5% CO2incubator.
Cell Proliferation Assays
Cells were seeded at 3,000 cells per well in growth media supplemented with 5% FBS, 2mmol/L L-glutamine, and 1% penicillin– streptomycin, allowed to adhere overnight, and treated with a dilution series of test compounds for 72 hours. Cell viability was determined by CellTiter-Glo, and results were represented as background-subtracted relative light units normalized to a dimethyl sulfoxide (DMSO)–treated control. Growth inhibition (GI50) values were determined by GraphPad Prism 5.04 (GraphPad Software). MK-2206 and XL-880 compounds were obtained from Selleck Chemical. CI data were generated using CalcuSyn.

-
动物实验
In vivo studies(compound 3 renamed CO-1686)[3]
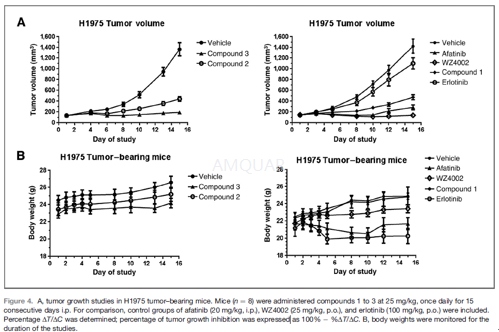
All the procedures related to animal handling, care, and the treatments in this manuscript were performed according to the guidelines approved by Institutional Animal Care and Use Committees (IACUC) following the guidance of the Association for Assessment and Accreditation of Laboratory Animal Care (AAALAC). Briefly, female nu/nu mice were implanted subcutaneously with 1 x 107H1975 tumor cells in 50% Matrigel (0.2mL injection volume) in the flank. Tumor measurements were recorded 3 times per week. Tumors were pair matched when they reached an average size of 100 to 150mm3. Group size was 8 mice. Test articles were administered intraperitoneally at 25 mg/kg daily for 15 days and control compounds were administered orally at indicated doses. Tumor volume was followed until tumors reached 1,500 mm3or until day 15.
For pharmacokinetic/pharmacodynamic studies, H1975 tumors in mice were allowed to reach ~500 mm3before being treated with test articles for 5 consecutive days. Tumors, plasma, and lung tissues were collected for pharmacokinetic and EGFR modulation analyses. Tumors and lungs were homogenized using 2.0 mL Precellys tubes for 30 seconds in a Precellys 24 Homogenizer (Precellys). Lysates were immunoblotted as described above.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Walter AO, Sjin RT, Haringsma HJ, et al. Discovery of a mutant-selective covalent inhibitor of EGFR that overcomes T790M-mediated resistance in NSCLC. Cancer Discov. 2013;3(12):1404-1415.
[2] Tjin Tham Sjin R LK, Walter AO, Dubrovskiy A, Sheets M, Martin TS, Labenski MT. In vitro and in vivo characterization of irreversible mutant-selective EGFR inhibitors that are wild-type sparing. Mol Cancer Ther. . 2014;13(6):1468-1479.
more
分子式
C27H28F3N7O3 |
分子量
555.55 |
CAS号
1374640-70-6 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
>90 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约28 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01526928 | Locally Advanced or Metastatic Non-small Cell Lung Cancer. | Drug: Rociletinib | Clovis Oncology, Inc. | Phase 1|Phase 2 | 2012-03-01 | 2016-07-26 |
| NCT02147990 | Non-small Cell Lung Cancer | Drug: Rociletinib | Clovis Oncology, Inc. | Phase 2 | 2014-04-01 | 2015-10-21 |
| NCT02547675 | Non-small Cell Lung Cancer | Drug: Rociletinib | Clovis Oncology, Inc. | | null | 2016-03-17 |
| NCT02322281 | Non-small Cell Lung Cancer | Drug: Rociletinib|Drug: Pemetrexed or gemcitabine or paclitaxel or docetaxel | Clovis Oncology, Inc. | Phase 3 | 2015-02-01 | 2016-06-02 |
| NCT02580708 | Non-small Cell Lung Cancer | Drug: Rociletinib|Drug: Trametinib | Clovis Oncology, Inc.|Novartis Pharmaceuticals | Phase 1|Phase 2 | 2015-09-01 | 2016-07-25 |
| NCT02630186 | Non-small Cell Lung Cancer | Drug: Rociletinib|Drug: MPDL3280A | Clovis Oncology, Inc.|Genentech, Inc. | Phase 1|Phase 2 | 2016-01-01 | 2016-05-17 |
| NCT02705339 | Carcinoma, Non-Small-Cell Lung|Non-Small Cell Lung Cancer|Nonsmall Cell Lung Cancer | Drug: Rociletinib|Procedure: Biopsy|Procedure: Blood draw | Washington University School of Medicine|Clovis Oncology, Inc. | Phase 2 | 2016-05-01 | 2016-05-16 |
| NCT02186301 | Non-Small Cell Lung Cancer | Drug: Rociletinib Mono-Therapy|Drug: Erlotinib Mono-Therapy | Clovis Oncology, Inc. | Phase 2|Phase 3 | 2014-11-01 | 2016-02-16 |
| NCT02108964 | Advanced Non-small Cell Lung Cancer (NSCLC) | Drug: EGF816 | Novartis Pharmaceuticals|Novartis | Phase 1|Phase 2 | 2014-06-01 | 2016-09-09 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们