-
生物活性
TAK-875 exhibits potent agonist activity and high binding affinity to the human GPR40 receptor with Ki of 38 nM. TAK-875 displays weaker affinity toward the rat GPR40 receptor with Ki of 140 nM. TAK-875 displays excellent selectivity, as TAK-875 has little agonist potency to other members of the FFA receptor family with EC50 of >10 μM. TAK-875 treatment induces a concentration-dependent increase in intracellular IP production in CHO-hGPR40 with EC50 of 72 nM, more potently than that of endogenous ligand agonist oleic acid which requires much higher ligand concentrations to activate the receptor with EC50 of 29.9 μM.
Agonist potency for human FFA receptor[1]
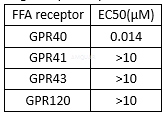
Agonist activity in CHO cells expressing human GPR40/FFAR1 (CHO-hGPR40): EC50= 0.072μM.[2]
Inhibition of mitochondrial respiration by TAK-875 in HepG2 cells[3]

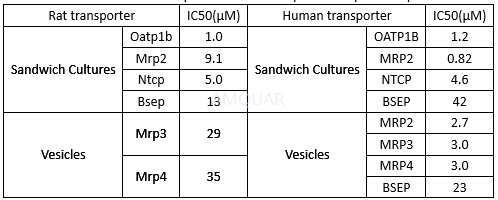
Inhibition of Rat and Human Uptake and Efflux Hepatic Transporters by TAK-875[3]
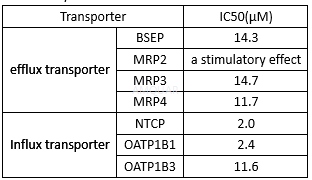
The ability of TAK-875 to inhibit the human version of bile acid (BA) transport proteins[4]
-
体外研究
-
体内研究
0.5% CMC+0.25% Tween 80
-
激酶实验
-
细胞实验
Cell Based Assays[4]
Single donor cryopreserved human hepatocytes (lot HH1031) were thawed in UCRM media (IVAL) and plated in HQM media (IVAL) at 25,000 cells/well in collagen coated, clear-bottom 96-well plates and incubated overnight at37OC with 5% CO2. Four hours after plating, the media was replaced with HQM that contained 0.1% DMSO or test compounds dissolved in DMSO. Duplicate cell cultures were treated with 0.1–100 μM of TAK-875 or TAK-875-Glu. For cell viability assays, the CellTiter-Glo kit was used to measure intracellular ATP after 6 or 24 h of incubation with the test compound. For glutathione quantification assays, the GSH-Glo kit was used after 6h of incubation. For both assays, luminescence was quantified using a Victor3 V Plate Reader. Data were analyzed in Prism 5, and the leastsquares method was used to determine the concentration causing lethality for 50% of cells (LC50).
A Seahorse XFe96 Analyzer was used to measure the mitochondrial effects of TAK-875 and TAK-875-Glu on hepatocytes. Cryopreserved primary human hepatocytes from a single donor were thawed and cultured on XF 96-well cell culture microplates in triplicate. The oxidative phosphorylation and glycolysis stress test mediawere prepared following manufacturer’s instructions using protein-free media. Cells were maintained in a CO2-free incubator for 1h at 37OC prior to measuring the oxygen consumption rate (OCR) and extracellular acidification rate (ECAR) 3 times over a 28 min span to establish a baseline. Compounds were added and the OCR and ECAR were measured in triplicate samples over a42 min period. Data was analyzed with the XF Wave (v 2.3) software. Results for the final measurement were normalized as a percentage of DMSO control wells.
Glucose and galactose (Gluc-Gal) assay
HepG2 human hepatocellular carcinoma cells were plated on a 96-well cell culture microplate in DMEM supplemented with 10% fetal bovine serum and cultured overnight. The next day, the medium was replaced with medium containing 25mM glucose or 10mM galactose. Cells were dosed in triplicate with either vehicle (0.5% DMSO) or a range of TAK-875 or TAK-875-Glu concentrations (in 0.5% DMSO) and incubated for 72 h at 37OC. Cells were stained with Hoechst 33342 and viability was determined with an automated fluorescent cellular imager, ArrayScan VTI.
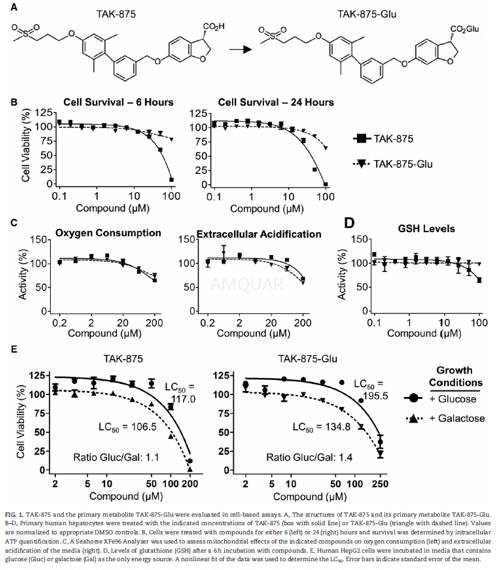
-
动物实验
Animals[5]
Male N-STZ-1.5 rats were prepared by administering120mg/kg of streptozotocin (STZ) subcutaneously to male Wistar Kyoto (WKY) rats on day 1.5 after birth. All rats were housed in individual metal cages in a room with controlled temperature (23°C), humidity (55%), and 12 hours dark/12 hours light. Rats were allowed free access to powdered standard laboratory chow diet and tap water.
Oral Glucose Tolerance Test (Mono-treatment)
At the age of20 to 27 weeks, N-STZ-1.5 and WKY rats underwent overnight fasting. N-STZ-1.5 rats were divided into 7 groups (n=6) so that plasma levels of glucose, triglycerides, and body weight were not statistically significantly different among groups. Each group of N-STZ-1.5 rats was given vehicle (0.5% methylcellulose), fasiglifam (3, 10, and30 mg/kg), or glibenclamide (3, 10, and 30 mg/kg) orally in a volume of 5 ml/kg. WKY rats (n=5) were given vehicle orally. Plasma parameters measured for the grouping of the animals were used as baseline data. Sixty minutes after drug administration, all animals received an oral glucose load (1.5 g/kg). Blood samples were collected from the tail vein just before (time 0) and 10, 30, 60, and 120 minutes after glucose load for the determination of plasma glucose and insulin levels.
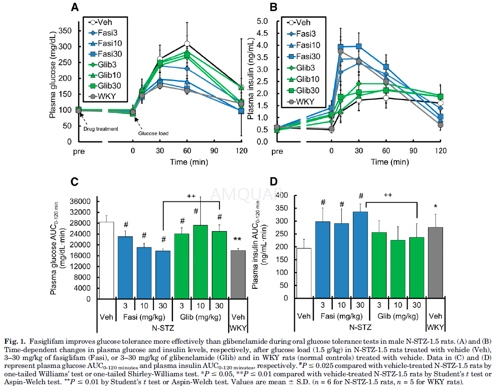
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Negoro N, Sasaki S, Mikami S, et al. Discovery of TAK-875: A Potent, Selective, and Orally Bioavailable GPR40 Agonist. ACS Med Chem Lett. 2010;1(6):290-294.
[2] Tsujihata Y, Ito R, Suzuki M, et al. TAK-875, an orally available G protein-coupled receptor 40/free fatty acid receptor 1 agonist, enhances glucose-dependent insulin secretion and improves both postprandial and fasting hyperglycemia in type 2 diabetic rats. J Pharmacol Exp Ther. 2011;339(1):228-237.
more
分子式
C29H32O7S.1/2H2O |
分子量
533.63 |
CAS号
1374598-80-7 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约28 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01609582 | Type 2 Diabetes|Cardiovascular Disease | Drug: TAK-875|Drug: TAK-875 Placebo | Takeda | Phase 3 | 2012-06-01 | 2015-10-15 |
| NCT01496443 | Pharmacokinetics | Drug: TAK-875 and Glimepiride | Takeda | Phase 1 | 2012-01-01 | 2012-09-12 |
| NCT01647542 | Type 2 Diabetes Mellitus | Drug: TAK-875|Drug: TAK-875 Placebo | Takeda | Phase 3 | 2012-10-01 | 2015-10-15 |
| NCT00949091 | Diabetes Mellitus, Type 2 | Drug: TAK-875 | Takeda | Phase 1 | 2009-01-01 | 2010-06-09 |
| NCT01829477 | Diabetes Mellitus, Type 2 | Drug: TAK-875|Drug: TAK-875 Placebo|Drug: Glimepiride | Takeda | Phase 3 | 2013-04-01 | 2016-04-24 |
| NCT01481116 | Diabetes Mellitus, Type 2 | Drug: TAK-875|Drug: TAK-875|Drug: Glimepiride | Takeda | Phase 3 | 2012-01-01 | 2016-04-24 |
| NCT01829464 | Diabetes | Drug: Placebo|Drug: TAK-875|Drug: TAK-875 | Takeda | Phase 3 | 2013-05-01 | 2016-04-24 |
| NCT02015780 | Type 2 Diabetes Mellitus|Chronic Kidney Disease | Drug: Fasiglifam|Drug: Placebo|Drug: Antihyperglycemic therapy | Takeda | Phase 3 | 2013-12-01 | 2014-01-24 |
| NCT01549964 | Glycemic Control | Drug: Fasiglifam (TAK-875)|Drug: Sitagliptin|Drug: Placebo | Takeda | Phase 3 | 2012-04-01 | 2016-04-20 |
| NCT01456195 | Diabetes Mellitus, Type 2 | Drug: Placebo|Drug: Fasiglifam | Takeda | Phase 3 | 2011-11-01 | 2016-03-04 |
| NCT01834274 | Diabetes Mellitus, Type 2 | Drug: Fasiglifam (TAK-875)|Drug: Fasiglifam (TAK-875) Placebo|Drug: Sitagliptin|Drug: Sitagliptin Placebo|Drug: Metformin | Takeda | Phase 3 | 2013-06-01 | 2015-08-26 |
| NCT01982253 | Type 2 Diabetes Mellitus | Drug: Fasiglifam|Drug: Placebo to fasiglifam | Takeda | Phase 2 | 2013-10-01 | 2016-08-15 |
| NCT01433419 | Diabetes Mellitus | Drug: TAK-875|Drug: TAK-875 | Takeda | Phase 3 | 2012-01-01 | 2013-03-21 |
| NCT01433406 | Diabetes Mellitus | Drug: TAK-875|Drug: TAK-875 | Takeda | Phase 3 | 2011-10-01 | 2014-01-24 |
| NCT01414920 | Diabetes Mellitus, Type 2 | Drug: Placebo|Drug: TAK-875|Drug: TAK-875|Drug: Sitagliptin|Drug: TAK-875 and Sitagliptin|Drug: TAK-875 and Sitagliptin | Takeda | Phase 2 | 2011-08-01 | 2016-03-04 |
| NCT01007097 | Diabetes Mellitus, Type 2 | Drug: TAK-875|Drug: TAK-875|Drug: TAK-875|Drug: TAK-875|Drug: TAK-875|Drug: Glimepiride|Drug: Placebo | Takeda | Phase 2 | 2009-12-01 | 2016-03-13 |
| NCT01433393 | Diabetes Mellitus | Drug: TAK-875|Drug: TAK-875|Drug: Placebo | Takeda | Phase 3 | null | 2012-11-07 |
| NCT01585792 | Diabetic Patients | Drug: TAK-875|Drug: TAK-875|Drug: Glimepiride|Drug: Placebo | Takeda | Phase 3 | 2012-05-01 | 2013-04-15 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们