-
生物活性
Pravastatin, Sodium Salt is a competitive HMGCR (HMG-CoA reductase) inhibitor that potently blocks cholesterol synthesis (Ki ~1 nM). Pravastatin offers cardioprotection and also acts as an immunomodulator and as an inhibitor of ras p21 isoprenylation.Water-soluble, competitive inhibitor of 3-hydroxy-3-methyl coenzyme A (HMG-CoA) reductase. Potently blocks cholesterol synthesis in vivo (Ki~ 1 nM) and displays cardioprotective properties.
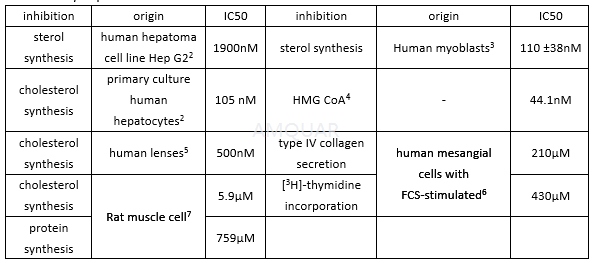
Pravastatin sodium, a hydrophilic inhibitor, inhibits sterol synthesis (HMG-CoA reductase activity) in lymphocytes with an IC50 of 5.6μM.[1]
The activity of pravastatin
-
体外研究
-
体内研究
-
激酶实验
Assay of microsomal HMG-CoA reductase activity[8]
The prepared microsomal pellet (5-10 mg of protein) was resuspended in 2-2.5 ml of buffer containing 20 mM imidazole-HCI (pH 7.4) and 5 mM dithiothreitol. The preincubation mixture contained the following concentrations of components in a volume of 90μl:20mM imidazole-HCl (pH 7.4), 5 mM dithiothreitol, 150μg of microsomal protein and 10 units of E. coil alkaline phosphatase. The tubes were incubated at 37 °C for 60 min, after which 90μlof a solution containing 0.2 M potassium phosphate (pH 7.4), 40 mM glucose-6-phosphate, 5 mM NADPH, 0.7 units of glucose-6-phosphate dehydrogenase, 20 mM NaEDTA and 10 mM dithiothreitol were added to the preincubation mixture. The HMG-CoA xeductase assay was then initiated with the addition of 20μl of DL-hydroxymethyl [3-14C]glutaryl-CoA solution to a final concentration of 175μM. After incubation for 30 rain at 37oC, the reaction was stopped by the addition of 0.2 ml of concentrated HCI. The tubes were incubated at 37°C for 30 min and then evaporated to dryness in a vacuum desiccator. The mevalonic acid lactone was dissolved in 200μlof acetone/H20 (9: 1, v/v) and isolated by thin-layer chromatography using Silica gel 60 plates (layer thickness 0.25 ram) and benzene/acetone (1 : 1, v/v) and the radioactivity was counted in liquid scintillation fluid.
-
细胞实验
Reagents[1]
Pravastatinsodium (salt form), simvastatin (lactone form), and simvastatin sodium (salt form; referred to as simvastatin-Na) were prepared. Since pravastatin-Na is used clinically in the active salt form, and simvastatin is used as the pro-drug lactone which, over a period of time, converts in vivo to its respective active salt form, both the lactone and salt form of simvastatin were prepared. The drugs were first solubilized in dimethylsulfoxide (DMSO) at 200μM concentration in RPMI 1640 medium with 10% fetal calf serum (FCS) and were further diluted to achieve the final concentrations just before use. DMSO never exceeded the toxic concentration of 0.05%. mevalonic acid.
Peripheral blood mononuclear cells (PBMCs)
PBMCs were separated from heparinized whole blood obtained from healthy volunteers by Ficoll-
Paque gradient centrifugatio. PBMCs were washed three times with phosphated-buffered saline and suspended in complete RPMI-1640 culture medium consisting of 10% FCS, 2 mM glutamine, 100 U/ml penicillin, and 100μg/ml streptomycin. Depletion of monocytes was performed by plastic adherence in RPMI- 1640 medium with 5% FCS and antibiotics.
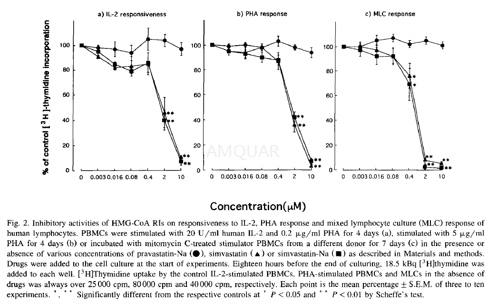
Proliferative response of PBMCs to interleukin 2 (IL-2) and phytohemagglutinin (PHA)
One line of approach to study the inhibitory activity of HMG-CoA RIs on T-cell responses would be to investigate proliferative responses of PBMCs to recombinant human IL-2 and T-cell mitogen PHA. The proliferative responses of PBMCs were performed using a standard [3H]thymidine uptake assay, and the experimental set up was as follows: PBMCs (1 X 105) were incubated for 4 days in the presence or absence of IL-2 (20 U/ml) with PHA (0.2μg/ml), or PHA (5μg/ml) in 0.2 ml of complete RPMI-1640 medium in quadruplicate in flat-bottomed microplates at 37°C in a humidified 5% CO2incubator. Eighteen hours before the end of culturing, 18.5kBq of [3H] thymidine (specific activity = 6.0TBq/mmol) was added to each well. The labelled cells were collected on a glass fibre filter, and the radioactivity trapped in the filter was counted using a liquid scintillation spectrometer.
-
动物实验
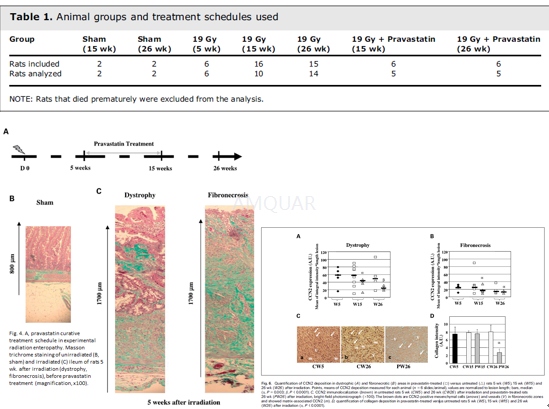
Animals and experimental procedures[9]
Male Wistar rats weighing 300 g at the beginning of the experimental period were obtained.
Rats were anaesthetized by inhaling an air/isoflurane mixture. A segment of the ileum was surgically exteriorized and irradiated with an X-ray machine operated at 225 kV and 17 mA with 0.5 mm copperadded filtration at a dose rate of 0.98 Gy/min. A single dose of 19 Gy was given locally on the ileum segment (6 cm), whereas the rest of the animal was shielded with a 5-mm-thick lead screen. The exteriorized segment was moistened with warm 0.9% sterile saline buffer over the course of the irradiation procedure. After irradiation, the exposed segment was returned to the abdominal cavity and the peritoneum, abdominal muscles, and skin were sutured separately. Fifty-four animals were divided into different groups (Table 1) and treated according to the schedule (see Fig. 4A). Radiation-induced fibrotic lesions were established 5 weeks after irradiation, this time was chosen as the starting time point for pravastatin treatment and continued for 10 weeks. A clinically relevant dose of 30 mg/kg/d was used as it is known to be the standard dose for the control of hypercholesterolemia in rats (in humans, the dose required for the control of hypercholesterolemia is 40 mg/d). Rats from each experimental group were anesthetized before intestinal sampling and formol fixation.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Kurakata S KM, Shimada Y, Komai T, Nomoto K. Effects of different inhibitors of 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) reductase, pravastatin sodium and simvastatin, on sterol synthesis and immunological functions in human lymphocytes in vitro. Immunopharmacology. 1996;34(1):51-61.
[2] Cohen LH vVA, Roodenburg L, Jansen LM, Griffioen M. Pravastatin inhibited the cholesterol synthesis in human hepatoma cell line Hep G2 less than simvastatin and lovastatin, which is reflected in the upregulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase and squalene synthase. Biochem Pharmacol. . 1993;45(11):2203-2208.
more
分子式
C23H35NaO7 |
分子量
446.51 |
CAS号
81131-70-6 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
88 mg/mL |
Water
90 mg/mL |
Ethanol
10 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01717586 | Preeclampsia | Drug: Pravastatin|Drug: Placebo | The University of Texas Medical Branch, Galveston|University of Texas|University of Pittsburgh|Northwestern University | Phase 1 | 2012-08-01 | 2016-07-15 |
| NCT01146093 | Healthy | Drug: Pravastatin Sodium | Dr. Reddy's Laboratories Limited | Phase 1 | 2002-11-01 | 2012-01-12 |
| NCT01146106 | Healthy | Drug: Pravastatin Sodium | Dr. Reddy's Laboratories Limited | Phase 1 | 2002-12-01 | 2010-06-16 |
| NCT00829309 | Healthy | Drug: Pravastatin|Drug: Pravastatin | Teva Pharmaceuticals USA | Phase 1 | 2005-03-01 | 2009-09-09 |
| NCT00830258 | Healthy | Drug: Pravastatin sodium 80 mg tablets|Drug: Pravachol庐 80 mg tablets | Teva Pharmaceuticals USA | Phase 1 | 2005-04-01 | 2009-09-09 |
| NCT00571194 | End Stage Renal Disease | Drug: pravastatin | Arkansas Children's Hospital Research Institute | Phase 1 | 2007-09-01 | 2017-01-26 |
| NCT01082588 | Schizophrenia|Schizoaffective Disorders|Schizophreniform Disorders | Drug: Pravastatin|Drug: Placebo | Massachusetts General Hospital|Stanley Medical Research Institute|North Suffolk Mental Health Association | Phase 4 | 2010-06-01 | 2014-03-26 |
| NCT00648544 | Therapeutic Equivalency | Drug: Pravastatin|Drug: Pravastatin | Mylan Pharmaceuticals|Genpharm ULC | Phase 1 | 2003-07-01 | 2008-03-31 |
| NCT00650221 | Therapeutic Equivalency | Drug: Pravastatin|Drug: Pravastatin | Mylan Pharmaceuticals|Genpharm ULC | Phase 1 | 2003-06-01 | 2008-03-31 |
| NCT02754739 | Diabetes Mellitus|Prediabetic State | Drug: Pravastatin|Drug: Placebo (for Pravastatin) | Samsung Medical Center|Daiichi Sankyo Korea Co., Ltd. | Phase 4 | 2012-09-01 | 2016-04-25 |
| NCT01497483 | Healthy | Drug: Pravastatin alone|Drug: Pravastatin and Cyclosporine | University of California, San Francisco|National Institute of General Medical Sciences (NIGMS) | Phase 1 | 2011-12-01 | 2015-01-14 |
| NCT00665717 | HIV Infections | Drug: Pravastatin|Drug: Raltegravir|Drug: Pravastatin and raltegravir | Radboud University|Merck Sharp & Dohme Corp. | Phase 1 | 2008-05-01 | 2011-06-06 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们