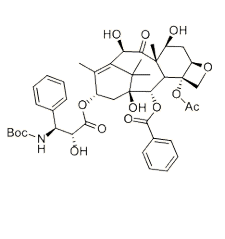
-
生物活性
Docetaxel is a semi-synthetic analog of the famous microtubule-stabilizing small molecule Taxol. These taxane compounds prevent the breakdown of microtubules necessary for mitotic progress, arresting the cell cycle at G2/M, leading to apoptosis. This cytotoxic activity has been widely exploited in suppressing oncogenic proliferation.Binds and stabilizes microtubules; leads to cell cycle arrest and apoptosis. Displays significant antitumor activity in ovarian and gastric tumors. Prevents microtubule depolymerization and disassembly in the absence of GTP. Exhibits cytotoxic activity.
Docetaxel increases the expression of E-cadherin and CEA in the low concentration range of 0.3 - 3 n M for HT-29 cells and 0.6 - 3 n M for SW620 cells.[10] Docetaxel inhibits the cell proliferation of HT-29 cells at an IC50 of 0.6n M and of SW620 cells at 0.8nM.[10]
Docetaxel inhibits HUVEC migration with an IC50 of 2pM.[9]
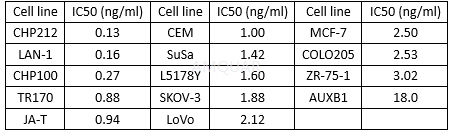
Cytotoxicity and anti-proliferative of docetaxel

IC50 values for docetaxel[15]
-
体外研究
-
体内研究
30% PEG400+0.5% Tween80+5% Propylene glycol
-
激酶实验
-
细胞实验
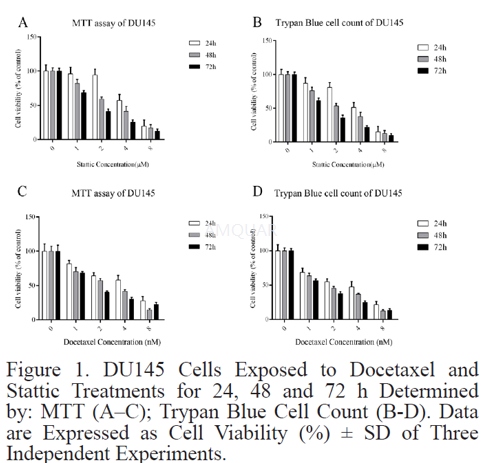
Cell culture[5]
Human prostate cancer (DU145) cell line was obtained and cultured under the condition of 37 °C, 5%
CO2, in RPMI 1640 Mediumwith 10% FBS (Fetal Bovine Serum) enriched with 100 units/ml streptomycin/ penicillin.
Cell viability assessment using MTT assay
Cells were seeded in the 96-well plate (2000 per well) in triplicate and treated with 1 to 16 nmol/L of docetaxel and 1 to 16 μmol/L of stattic for 24, 48 and 72 hours. The media in each well was replaced with 200 μl fresh media containing 20 μL of MTT (Methylthiazolyldiphenyltetrazolium bromide) solution (2 mg/ml). Then; the cells were incubated for 4 hours at 37 °C. Subsequently, media/MTT mixture was removed and 200μl of DMSO (Dimethyl sulfoxide) plus 25μl of Sorenson’s glycine buffer as solubilization solution was added to each well. The absorbance at 570 nm was read after shaking for 30 minutes, employing a microplate reader (Awareness, statfax 3200, USA). Half maximal inhibitory concentration (IC50) was calculated using Graphpad prism 6.07 software.
Cell viability analysis using trypan blue staining
Trypan Blue is a “vital stain” in which dead cells were excluded from live cells. Live cells appear colorless
and bright (retractile) while dead cells stain blue and are non-retractile. Cells were re-suspended with equal volumes of 0.4% trypan blue stain for 3 min. Then, the cells were counted by hemocytometer. The number of cells from the untreated control represented optimal cell survival (100%) and the relative surviving cells of each treatment were calculated usingthe following equation:Relative cell viability (%) =no. surviving cells in control / no. surviving cells in sample x100%
The experiments were repeated three times separately, to confirm reproducibility.
-
动物实验
In vivo experiments[3]
Male SCID mice (5-6 weeks old, 20-25 g) were purchased and kept under sterile and standardized environmental conditions. All experiments were carried out according to the animal protection law with permission from the responsible local authorities. For testing toxicity, groups of 3 animals each were treated with single doses of 2, 4, 8, and 16 mg/kg bw DOC i.p. or 0.15, 0.3, 0.6, 1.2, and 2.4 mg/kg bw D7(VLVH)-PE40 i.v. Mice were observed for apparent signs of toxicity (loss of weight and appetite, changes in pelage, fever, tension, apathy, aggression, respiratory disorders, paralyses, death) over a period of 14 days. The maximal dose of each substance, which was tolerated without any signs of toxicity, was defined as the maximal tolerable dose (MTD). After MTD determination of DOC and D7(VL-VH)-PE40, a toxicity experiment was performed treating additional three animals with repeated DOC and immunotoxin doses according to the therapy plan in the next paragraph.
For antitumor therapy, SCID mice were subcutaneously injected with 2.5 x 106C4-2 cells in 100μl PBS mixed with 100 μl Matrigel into the right flank (day 1 of treatment). Growing tumors were palpated and tumor diameters were measured in two axes using a vernier caliper. The mean radius (r) was determined to calculate the tumor volume using the formula V=4/3 πr3. When tumors reached volumes of about 20 mm3at day 14 of treatment, mice were randomized into four groups. The first group (n = 10) received combinatorial treatment with one dose of DOC (0.5 x MTD, i.p.) at day 15, followed by 3 doses of D7(VL-VH)-PE40 (0.5 MTD each, i.v.) at the days 16, 18 and 20 of treatment. The second group (n = 10) was injected with DOC alone and the third one (n = 10) with D7(VL-VH)-PE40 alone. The fourth group of mice (n = 9) received PBS injections as control. During the experiment, tumor sizes and body weights (bw) of the animals were measured 3 to 4 times a week. At day 29 of the experiment, mice were euthanized. For each animal in each group the time-adjusted Area-Under-the-Curve (AUC) was calculated. To estimate the inhibition effect, the ratio of the means for treatment and control (TCR) was determined. A significant inhibition effect can be claimed, if the upper limit of Fieller's one-sided 95%-confidence interval is below 1.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Batista de Carvalho AL, Medeiros PS, Costa FM, et al. Anti-Invasive and Anti-Proliferative Synergism between Docetaxel and a Polynuclear Pd-Spermine Agent. PLoS One. 2016;11(11):e0167218.
[2] Mahsa Mohseni NS, Parisa Ghanbari , Bahman Yousefi , Maryam Tabasinezhad , Simin Sharifi , Hossein Nazemiyeh. Co-treatment by docetaxel and vinblastine breaks down P-glycoprotein mediated chemo-resistance. Iran J Basic Med Sci. 2016;19(3):300-309.
more
分子式
C43H53NO14 |
分子量
807.88 |
CAS号
114977-28-5 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
25 mg/mL |
Water
Insoluble |
Ethanol
25 mg/mL |
体内溶解度
约28 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01110291 | CYP3A Phenotyping|CYP3A5 and MDR1 Genotyping|Docetaxel Toxicity|Associations Between Genetic Data and Docetaxel Toxicity | Drug: docetaxel + CEF | University of Turku|Sanofi|Turku University Hospital|Vaasa Central Hospital, Vaasa, Finland|medbase Oy Ltd | | 2003-04-01 | 2010-04-26 |
| NCT00675545 | Hormone-Refractory Prostate Cancer | Drug: Docetaxel, Carboplatin | National University Hospital, Singapore | Phase 2 | 2007-05-01 | 2012-03-30 |
| NCT00630110 | Cancer | Drug: docetaxel|Drug: NPI-2358 + docetaxel | Nereus Pharmaceuticals, Inc. | Phase 1|Phase 2 | 2008-02-01 | 2011-08-15 |
| NCT00795171 | Hormone Refractory Prostate Cancer | Drug: Docetaxel * Sunitinib|Drug: Docetaxel|Drug: Docetaxel | Medical University of Vienna | Phase 2 | 2008-11-01 | 2010-08-17 |
| NCT00606814 | Solid Tumors | Drug: IPI-504, docetaxel | Infinity Pharmaceuticals, Inc. | Phase 1 | 2007-12-01 | 2012-03-02 |
| NCT01949519 | Adenocarcinoma of the Prostate | Drug: Lycopene and Docetaxel | Medical University of South Carolina|National Cancer Institute (NCI) | Phase 1 | 2013-11-01 | 2016-10-18 |
| NCT00703378 | Solid Tumors | Drug: Docetaxel (Taxotere庐) | National University Hospital, Singapore | Phase 1|Phase 2 | 2006-05-01 | 2012-03-27 |
| NCT01837667 | Tumors|Neoplasms|Cancer | Drug: LB-100 for Injection|Drug: Docetaxel | Lixte Biotechnology Holdings, Inc. | Phase 1 | 2013-02-01 | 2017-01-23 |
| NCT01254513 | Prostate Cancer | Drug: Docetaxel every 3 weeks + Prednisone|Drug: Docetaxel weekly+ Prednisone | UNICANCER | Phase 2 | 2010-11-01 | 2013-01-29 |
| NCT00248703 | Breast Cancer | Drug: Docetaxel | Oslo University Hospital|University of Tromso|Helse Stavanger HF|Sorlandet Hospital HF|Sykehuset Innlandet HF|Ullevaal University Hospital|Sykehuset i Vestfold HF|Sykehuset Ostfold|Alesund Hospital | Phase 2 | 2003-10-01 | 2012-11-20 |
| NCT00440128 | Nausea and Vomiting, Chemotherapy-Induced | Drug: Docetaxel|Drug: Casopitant/Docetaxel | GlaxoSmithKline | Phase 1 | 2007-05-01 | 2016-11-02 |
| NCT00030407 | Lung Cancer | Drug: Celecoxib|Drug: Docetaxel | Barbara Ann Karmanos Cancer Institute|National Cancer Institute (NCI) | Phase 2 | 2001-10-01 | 2013-04-25 |
| NCT00169000 | Metastatic Breast Cancer | Drug: Capecitabine|Drug: Docetaxel | Dartmouth-Hitchcock Medical Center|Hoffmann-La Roche | Phase 1 | 2003-01-01 | 2009-08-04 |
| NCT00935961 | HEAD & NECK Cancer | Drug: RAD001 + docetaxel + cisplatin | Memorial Sloan Kettering Cancer Center|Novartis Pharmaceuticals | Phase 1 | 2009-07-01 | 2013-08-07 |
| NCT00995761 | Non-small Cell Lung Cancer | Drug: Docetaxel and Cisplatin | Gyeongsang National University Hospital | Phase 2 | 2009-10-01 | 2014-02-09 |
| NCT00294385 | Breast Cancer | Drug: Docetaxel|Drug: Gemcitabine, Docetaxel|Drug: Docetaxel | Central European Cooperative Oncology Group | Phase 3 | 2002-06-01 | 2011-06-22 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们