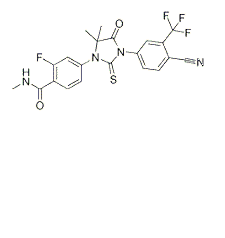
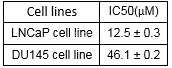
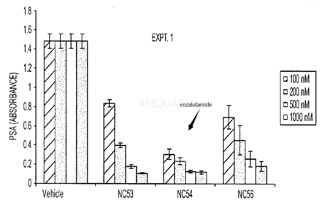
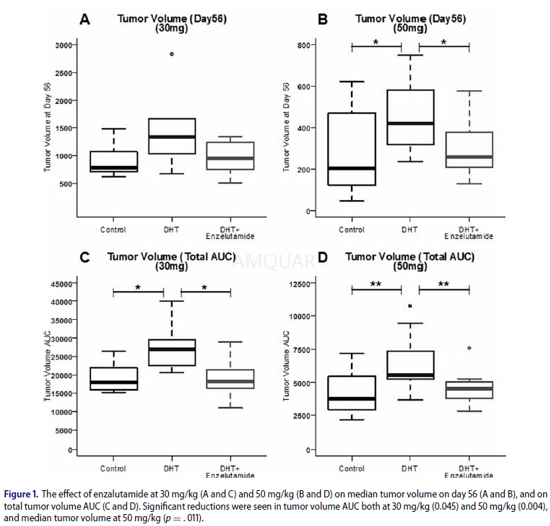
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01902251 | Prostate Cancer|Pharmacokinetics of Enzalutamide | Drug: Enzalutamide tablet|Drug: Enzalutamide capsule | Astellas Pharma Europe B.V.|Medivation, Inc.|Astellas Pharma Inc | Phase 1 | 2012-11-01 | 2014-09-08 |
| NCT01927627 | Adenocarcinoma of the Prostate | Drug: enzalutamide | Case Comprehensive Cancer Center | Phase 2 | 2013-11-01 | 2016-09-15 |
| NCT02124668 | Castration-Resistant Prostate Cancer|Prostate Cancer | Drug: Enzalutamide | Astellas Pharma Europe B.V.|Medivation, Inc.|Astellas Pharma Inc | Phase 2 | 2014-09-01 | 2016-12-22 |
| NCT02953860 | Breast Cancer | Drug: Fulvestrant with Enzalutamide | University of Colorado, Denver|United States Department of Defense | Phase 2 | 2017-01-01 | 2017-01-26 |
| NCT02500901 | Metastatic Prostate Cancer | Drug: Enzalutamide|Drug: Niraparib | Paul Mathew, MD|Hoosier Cancer Research Network|Tesaro, Inc. | Phase 1 | 2016-03-01 | 2017-02-06 |
| NCT02138162 | Severe Hepatic Impairment|Normal Hepatic Function | Drug: enzalutamide | Astellas Pharma Europe B.V.|Medivation, Inc.|Astellas Pharma Inc | Phase 1 | 2013-08-01 | 2014-05-12 |
| NCT01911741 | Healthy Subjects|Castration Resistant Prostate Cancer (CRPC)|Relative Bioavailability|MDV3100 | Drug: MDV3100 | Astellas Pharma Europe B.V.|Medivation, Inc.|Astellas Pharma Inc | Phase 1 | 2012-11-01 | 2013-07-26 |
| NCT02605863 | Bladder Cancer | Drug: Enzalutamide | University of Rochester|Astellas Pharma Inc | Phase 2 | 2016-01-01 | 2017-01-12 |
| NCT02138799 | Drug-Drug Interaction (DDI)|Healthy Subjects|Pharmacokinetics of Enzalutamide | Drug: enzalutamide|Drug: rifampin | Astellas Pharma Europe B.V.|Medivation, Inc.|Astellas Pharma Inc | Phase 1 | 2013-07-01 | 2014-10-14 |
| NCT02028988 | Intermediate Risk Prostate Cancer | Drug: Enzalutamide|Radiation: External Beam Radiation | Dana-Farber Cancer Institute|Medivation, Inc. | Phase 2 | 2014-01-01 | 2017-02-13 |
| NCT02684227 | Endometrial Cancer | Drug: Enzalutamide|Drug: Carboplatin|Drug: Paclitaxel | M.D. Anderson Cancer Center|Astellas Pharma Inc|Medivation, Inc.|National Comprehensive Cancer Network | Phase 2 | 2016-08-01 | 2016-12-01 |
| NCT02384382 | Prostate Carcinoma Metastatic to the Bone|Castration Resistant Prostate Cancer | Drug: Enzalutamide | Medivation, Inc.|Astellas Pharma Inc | Phase 2 | 2015-11-01 | 2017-01-18 |
| NCT02288936 | Hormone-refractory Prostate Cancer | Drug: Enzalutamide | Spanish Oncology Genito-Urinary Group | Phase 2 | 2015-02-01 | 2017-02-28 |
| NCT02207504 | Castration-resistant Prostate Cancer | Drug: Crizotinib|Drug: Enzalutamide | Dana-Farber Cancer Institute|Astellas Pharma Inc|Pfizer | Phase 1 | 2014-08-01 | 2017-01-29 |
| NCT01302041 | Prostate Cancer | Drug: Enzalutamide | Astellas Pharma Inc|Medivation, Inc. | Phase 2 | 2011-05-01 | 2017-01-10 |