-
生物活性
Lapatinib inhibits the tyrosine kinase activity associated with two oncogenes, EGFR (epidermal growth factor receptor) and HER2/neu (human EGFR type 2). Over expression of HER2/neu can be responsible for certain types of high-risk breast cancers in women.Like sorafenib, lapatinib is a protein kinase inhibitor shown to decrease tumor-causing breast cancer stem cells. Lapatinib inhibits receptor signal processes by binding to the ATP-binding pocket of the EGFR/HER2 protein kinase domain, preventing self-phosphorylation and subsequent activation of the signal mechanism.
Enzyme inhibition[1]

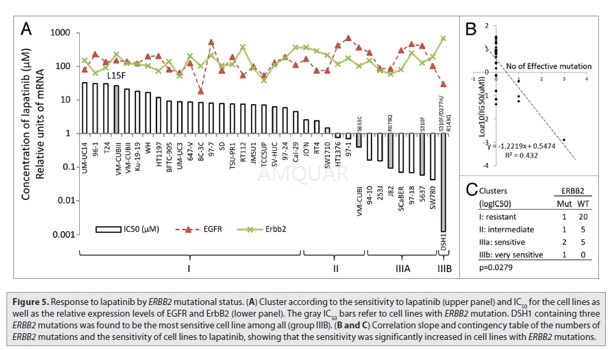
The antiproliferative activity of Lapatinib in breast cancer cell lines[2]

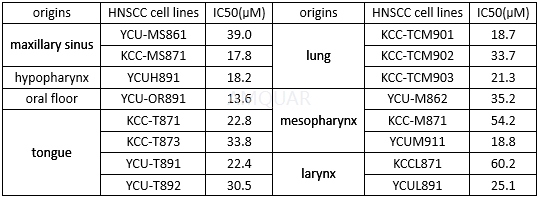
The antiproliferative activity of lapatinib in 16 HNSCC cell lines[3]
-
体外研究
-
体内研究
-
激酶实验
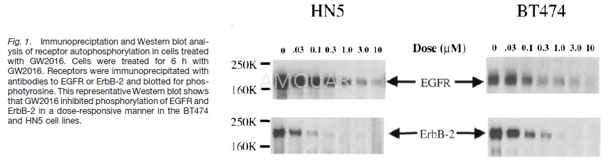
In Vitro Kinase Assays[1]
The intracellular kinase domains of EGFR, ErbB-2, and ErbB4 were purified from a baculovirus expression system. EGFR, ErbB-2, and ErbB-4 reactions were performed in 96-well polystyrene round-bottomed plates in a final volume of 45 μl. Reaction mixtures contained 50 mM 4-morpholinepropanesulfonic acid (pH 7.5), 2 mM MnCl2, 10 μM ATP, 1 μCi of [γ-33P] ATP/reaction, 50 μM Peptide A [Biotin-(amino hexonoic acid)-EEEEYFELVAKKKCONH2; Quality Controlled Biochemicals, Inc.], 1 mM dithiothreitol, and 1 μl of DMSO containing serial dilutions of GW2016 beginning at 10 μM. The reaction was initiated by adding the indicated purified type-1 receptor intracellular domain. The amount of enzyme added was 1 pmol/reaction (20 nM). Reactions were terminated after 10 min at 23°C by adding 45 l of 0.5% phosphoric acid in water. The terminated reaction mix (75μl) was transferred to phosphocellulose filter plates (Millipore, Marlborough, MA). The plates were filtered and washed three times with 200 μl of 0.5% phosphoric acid. Scintillation cocktail (50 μl, Optiphase; Wallac) was added to each well, and the assay was quantified by counting in a Packard Topcount.
-
细胞实验
Cell culture[4]
All cells were maintained in Roswell Park Memorial Institute medium 1640 (RPMI-1640 medium) or Dulbecco’s modified Eagle medium, which were supplemented with 10% fetal bovine serum and 1% non-essential amino acids. Cells were grown for one week in a humidified incubator at 37 °C and 5% CO2before the experiment, and planted at a density of 1000 cells/well in a 96-well plate.
Response to lapatinib
Microtetrazolium (MTT) assays were performed to assess the concentration of lapatinibnecessary to inhibit cell viability of 50%. Briefly, cells were treated for 48 h with different concentration of reagents ranging from 0.01 to 100 μM. Graphpad Prism software was used to calculate the IC50 value which was found to be 0.75 μM for lapatinib. The infected Bladder cancer cell lines(RT112 cells ) were cultured for 7 d and subsequently incubated with 0.75 μM lapatinib. As control, there were 5 wells with medium only and 5 wells with 0.1% DMSO for each plate. After 48 h of incubation, the presence of mini-foci was evaluated under the microscope. The relative numbers of viable cells were assessed before and after treatment using Celltiter Blue® Cell Viability Assay (Promega).
-
动物实验
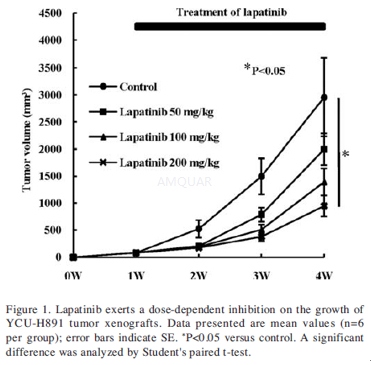
In vivo antitumor activity studies[3]
Female BALB/c nu/nu nude mice, 6-week old, were obtained. The mice were maintained in a laminar flow room with a constant temperature and humidity. Suspensions of YCU-H891 cells (100 μl) (final concentration, 1x107 cells/100 μl) were injected s.c. into the right flank of the mice on day 1. Tumor-bearing mice were randomized (n=6) when the mean tumor volume was 50-100 mm3. Each group was closely matched before treatment, which began one week after cell transplantation. Lapatinib was dissolved in 0.5% hydroxypropylmethylcellulose and 0.1% Tween-80 vehicle and was given once daily by oral gavage. The mice were treated with lapatinib (50, 100, 200 mg/kg) from day 8 to day 28. The control group of mice received hydroxypropylmethylcellulose/Tween-80 vehicle treatment at the same schedule as lapatinib. Treatment group number was 8. Tumor diameters in the control and treated groups were measured weekly with a Vernier caliper. Tumor volume (V) was determined by the equation: V=ab2/2 (a, length; b, width).
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] David W. Rusnak KL, Karen Affleck. The Effects of the Novel, Reversible Epidermal Growth Factor Receptor/ErbB-2 Tyrosine Kinase Inhibitor, GW2016, on the Growth of Human Normal and Tumor-derived Cell Lines in Vitro and in Vivo. Molecular Cancer Therapeutics. 2001;1(2):85-94.
[2] Hegde PS, Rusnak D, Bertiaux M, et al. Delineation of molecular mechanisms of sensitivity to lapatinib in breast cancer cell lines using global gene expression profiles. Mol Cancer Ther. 2007;6(5):1629-1640.
more
分子式
C29H26ClFN4O4S |
分子量
581.06 |
CAS号
231277-92-2 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
100 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约29 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT01184482 | Colorectal Cancer|Lung Cancer|Head and Neck Cancer | Drug: cetuximab and lapatinib | Georgetown University|GlaxoSmithKline | Phase 1 | 2010-06-01 | 2013-10-29 |
| NCT00650910 | Metastatic ErbB2|Neoplasms, Breast|Breast Cancer | Drug: lapatinib|Drug: Digoxin | GlaxoSmithKline | Phase 1 | 2008-04-01 | 2012-05-31 |
| NCT00694252 | Metastatic Breast Cancer | Drug: Lapatinib | University Hospital of Crete | Phase 2 | 2008-07-01 | 2015-09-25 |
| NCT00820924 | Neoplasms, Breast | Drug: LAPATINIB | GlaxoSmithKline | Phase 2 | 2008-06-01 | 2012-03-22 |
| NCT00863122 | Vestibular Schwannoma|NF2|Neurofibromatosis 2|Acoustic Neuroma|Auditory Tumor | Drug: lapatinib | Sidney Kimmel Comprehensive Cancer Center|The Children's Tumor Foundation|GlaxoSmithKline|New York University|Ohio State University|House Research Institute|Washington University School of Medicine|Weill Medical College of Cornell University|Massachusetts General Hospital | Early Phase 1 | 2009-06-01 | 2013-08-05 |
| NCT01290354 | Cancer | Drug: Lapatinib|Drug: [11C] lapatinib | GlaxoSmithKline | Phase 1 | 2011-09-01 | 2013-08-01 |
| NCT00614978 | Metastatic Breast Cancer|Brain Metastases|HER2 Positive | Drug: lapatinib and temozolomide | Jules Bordet Institute|GlaxoSmithKline|Schering-Plough | Phase 1 | 2008-01-01 | 2012-09-18 |
| NCT00912275 | Metastatic Breast Cancer | Drug: lapatinib plus oral vinorelbine | National Taiwan University Hospital|GlaxoSmithKline | Phase 1|Phase 2 | 2009-05-01 | 2012-11-15 |
| NCT00496366 | Breast Cancer|Metastatic Breast Cancer|Advanced Breast Cancer|HER2/Neu-positive Breast Cancer | Drug: Capecitabine|Drug: Lapatinib | Rutgers, The State University of New Jersey|National Cancer Institute (NCI)|Rutgers Cancer Institute of New Jersey | Phase 2 | 2007-07-23 | 2017-03-20 |
| NCT00225758 | Metastatic Breast Cancer | Drug: Lapatinib | Dartmouth-Hitchcock Medical Center|University of Colorado, Denver|North Shore University Hospital | Phase 2 | 2006-01-01 | 2015-01-12 |
| NCT01454479 | Recurrent Endometrial Cancer | Drug: Lapatinib and ixempra | Hung-Hsueh Chou|GlaxoSmithKline|Bristol-Myers Squibb|Chang Gung Memorial Hospital | Phase 1 | 2011-03-01 | 2011-10-18 |
| NCT00528281 | Advanced Non-Small Cell Lung Cancer|Lung Cancer, Non-Small Cell | Drug: Lapatinib|Drug: Pemetrexed | GlaxoSmithKline | Phase 1 | 2007-09-01 | 2012-05-31 |
| NCT01283789 | Metastatic Breast Cancer | Drug: Lapatinib and RAD-001 | University of Kansas Medical Center|Novartis|GlaxoSmithKline | Phase 2 | 2011-02-01 | 2017-02-06 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们