-
生物活性
Ibrutinib(PCI-32765) is a potent and highly selective Bruton tyrosine kinase (BTK)inhibitor.[1]
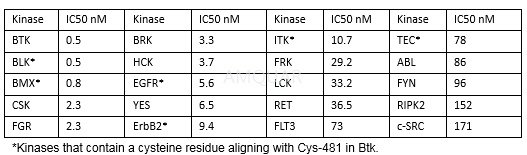
Antiproliferativeactivity (IC50)[12][8]: Ramos 12.6 μM; Raji 19.3 μM; BCR-activated primary B cell 8 nM.
Inhibition of productionin primary monocytes (IC50)[8]: TNFα 2.6 nM; IL-1b 0.5 nM; IL-6 3.9 nM.
-
体外研究
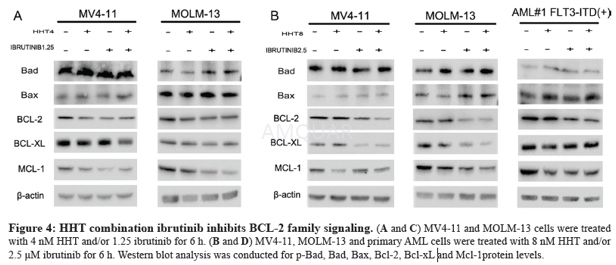
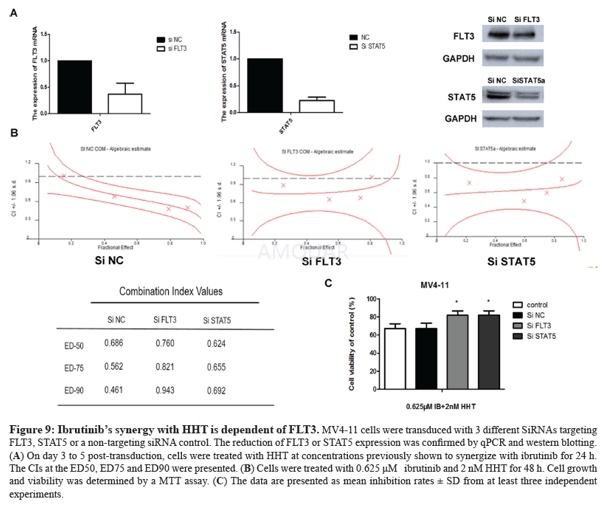
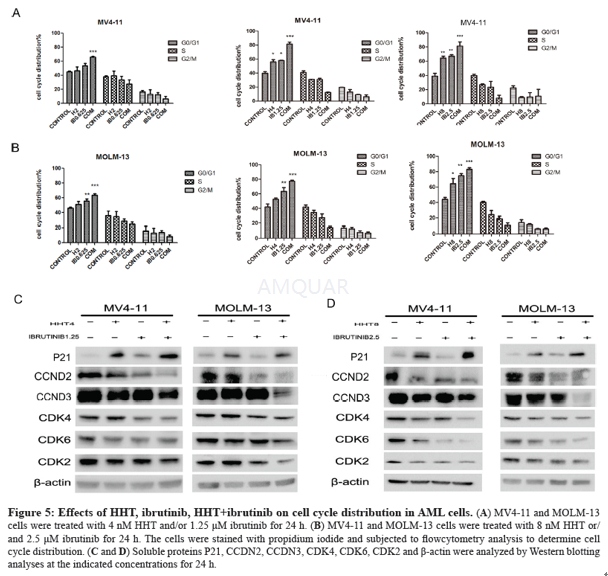
Ibrutinibinhibits BCR signaling downstream of Btk, selectively blocks B-cell activation,and is efficacious in animal models of arthritis, lupus, and B-cell lymphoma. Besides,Ibrutinib blocks BCR signaling in human peripheral B cells at concentrationsthat did not affect T cell receptor signaling.[1] Byirreversibly binding to the active site of ITK, thus inhibiting its activity, ibrutinibdrives CD4 T-cell populations toward a Th1 profile, which could potentiallyenhance tumour immune surveillance.[2] Besides, Ibrutinib inhibits ITK‘s downstream target PLCγ1in T-cells as an off-target effect. This may have great off-target effects onSRC kinase inhibition in healthy T cells with an EC50 less than 0.2 μM. Ibrutinibreduced phosphorylation of ITK and its substrate, PLCγ1 at low (0.25 and 0.5μM) concentrations.[3] Ibrutinibinhibits downstream signal transduction/survival pathways (such as MAPK, PI3Kand NF-КB), as indicated by its ability to reduce phosphorylation of PLCƳ, ERKand AKT and the nuclear expression of NF-КB p50. BCR and NF-jB signalling isinhibited rapidly by ibrutinib in CLL cells, regardless of whether the cellsare in the peripheral blood or resident in the lymph nodes/bone marrow.[4][5] For stimulation with anti-IgE, Ibrutinibinhibited CD63, histamine, LTC4 and IL-4 secretion with an IC50 of 3–6 nM (with100% inhibition at 50 nM) and it inhibited CD203c and CD11b and the cytosoliccalcium response with and IC50 of 30–40 nM.[6] Ibrutinib shows an inhibitory effect onFLT3-ITD mutant AML cells by regulating the STAT5/Pim-2/C-Myc pathway, AKTpathway and Bcl-2 family, activating p21WAF1/CIP1 and inhibiting CCND/CDKcomplex protein when combined with HHT.[7] Invitro, Ibrutinib inhibited BCR-activated primary B cell proliferation (IC50 = 8nM). Following FcƳR stimulation, Ibrutinib inhibited TNFα, IL-1b and IL-6production in primary monocytes (IC50 = 2.6, 0.5, 3.9 nM, respectively).Following FcƳR stimulation of cultured human mast cells, Ibrutinib inhibitedrelease of histamine, PGD2, TNF-α, IL-8 and MCP-1.[8] Ibrutinibis an effective inhibitor of ErbB receptor phosphorylation and of the viabilityof ErbB2+ breast cancer cells, suggesting therapeutic use of Ibrutinib inbreast cancer.[9] Ibrutinib may act directly on CLL cells toinduce apoptosis, but may also act on the surrounding microenvironment toinhibit external sources of proliferation and also help prevent the creation ofa survival niche for the CLL cells.[4] Ibrutinibappears to alter the broader immune microenvironment and disrupt interactions/signalsthat may protect and promote CLL cell survival and migration.
-
体内研究
2% DMSO+Castor oil
-
激酶实验
In vitro kinase enzymatic assay
The BTK kinaseenzyme system (Catalog. V9071) was purchased from Promega Corporation (USA).Concentrations consisting of suitable levels from 0.1 to 100 nM were used forall of the tested compunds. The experiments were performed according to theinstructions of the manufacturer. The testwas performed in a 384-well plate, and includes the major steps below: (1)perform a 5 μL kinase reaction using 1 X kinase buffer (e.g., 1 Xreaction buffer A), (2) incubate at room temperature for 60 min, (3) add 5 μL of ADP-Glo™Reagent to stop the kinase reaction and deplete the unconsumed ATP, leavingonly ADP and a very low background of ATP, (4) incubate at room temperature for40 min, (5) add 10 μL of Kinase Detection, (6) reagent toconvert ADP to ATP and introduce luciferase and luciferin to detect ATP, (7) incubateat room temperature for 30 min, (8) plate was measured on TriStar® LB942Multimode Microplate Reader (BERTHOLD) to detect the luminescence (Integrationtime 0.5-1 s). Curve fitting and data presentations were performed usingGraphPad Prism version 5.0. 25 - 27.[12]
-
细胞实验
Cell lines and primary cells[7]
The FLT3-ITD+ AMLcell lines MV4-11 and MOLM-13 were kindly endowed by Professor Ravi Bhatia(City of Hope National Medical Center, Duarte, CA). These two cell lines werecultured in IMDM medium supplemented with 10% fetalbovine serum at 37 °C in a humidified incubator containing 5% CO2.Bone-marrow samples were obtained from AML patients after obtaining writteninformed consent. Peripheral blood mononuclear cells (PBMCs) were isolated byFicoll-Hypaque (Sigma–Aldrich) density gradient centrifugation. The mutationsin FLT3 internal tandem duplication (FLT-3ITD) were tested by the FirstAffiliated Hospital (Zhejiang University College of Medicine). The study wasapproved by the Ethics Committee of the First Affiliated Hospital, College ofMedicine, Zhejiang University (Hangzhou, China).
Growth inhibition assay
Cells were seededin 96-well plates at 1 × 105 (cell-line cells) or 5 × 105 (primary AML cells).After treated with different drugs for 24 h, 20 μL MTT solution (5 mg/mL) was added to each well and thecells were incubated for an additional 4h at 37 °C .The cell medium was thenremoved and 200 ul DMSO was added to the 96-well plates to dissolve the MTTcrystals. The plates were read at an absorbance of 570 nm. Cell-linesexperiments were repeated three times.
Apoptosisassay
Cells were treated with drugs for 24 h and 48 h andthen harvested. After washed twice with phosphate buffered saline (PBS), cellswere resuspended in binding buffer. Cells were then co-stained with 5 μL AnnexinV-Fluorescein Isothiocyanate (FITC) and 5 μL Propidium Iodide (PI) using an apoptosis detection kit. The apoptic cells were analyzed by FACScan flow cytometer.
Cellcycle analysis
Cells were treated with drugs for 24 h. At the endof the treatment, the cells were washed with PBS and fixed with 75% ethanol at 4 °C. The next day, the cells were harvested and washed with PBS twice thenresuspended in buffer with 50 μg/ml PI (propidium iodide) and 100 μg/ml RNase Afor 30 min. The DNA content was analysed by FACScanflow cytometer.
Cell culture[12]
Ramos and Rajicells were purchased from Fuheng Biology Company (Shanghai, China). Peripheralblood mononuclear (PBMC) cells were obtained from a healthy adult male. Allcell lines were grown in RPMI-1640 supplemented with 10% FBS, 1% penicillinstreptomycin. The cellswere maintained and propagated as monolayer cultures at 37 oC in humidified 5% CO2 incubator.
Cellular activity assay
All the cellviability assays were performed according to the CCK-8 method. The cells wereseeded in 96-well plates at a density of 2000 to 3000 cells/well and weremaintained at 37 ℃ in a 5% CO2 incubator in RPMI1640 containing 10%fetal bovine serum for one day. Cells were exposure to treatmentfor 48 h, and the number of cells used per experiment for each cell lines was adjustedto obtain an absorbance of 0.5e1.2 at 450 nm with a microplate reader. Compounds were tested at appropriate concentrations (1.25e40 mM), with eachconcentration duplicated five times. The IC50 values were calculated using GraphPadPrim version 5.0.
-
动物实验
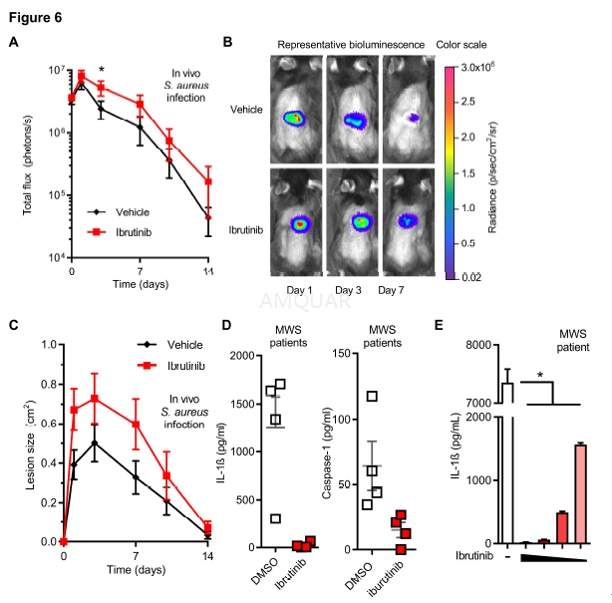
Ibrutinib (6 mg/kg)in 3% DMSO and 5% corn oil in PBS or vehicle control was injected i.v. via theretro-orbital vein in anesthetized mice on days -1, 0, 1, and topically on day2 (6 mg/kg ibrutinib in 10 μL DMSO or vehicle control). On day 0 the dorsalbacks of anesthetized mice (2% isoflurane) were shaved, injected intradermallywith 3x107 CFU of S. aureus LAC::Lux: digitally imaged on 1, 3,7, 10 and 14, and total lesion size (cm2 ) analyzed using ImageJwith a millimeter ruler as a reference. Mice were anesthetized (2% isoflurane)and in vivo 252 bioluminescent imaging was performed (Lumina III IVIS,PerkinElmer) and total flux (photons/s) within a circular region of interestmeasuring 1x103 253 pixels was measured using Living Image software(PerkinElmer) (limit of detection: 2x104 254 photons/s).[13]
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Honigberg LA, Smith AM, Sirisawad M, et al. The Bruton tyrosine kinase inhibitor PCI-32765 blocks B-cell activation and is efficacious in models of autoimmune disease and B-cell malignancy. Proc Natl Acad Sci U S A. 2010;107(29):13075-13080.
[2] Dubovsky JA, Beckwith KA, Natarajan G, et al. Ibrutinib is an irreversible molecular inhibitor of ITK driving a Th1-selective pressure in T lymphocytes. Blood. 2013;122(15):2539-2549.
more
分子式
C25H24N6O2 |
分子量
440.5 |
CAS号
936563-96-1 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
>80 mg/mL |
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
约10.5 mg/mL
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02955628 | Diffuse Large B Cell Lymphoma|Diffuse Large B-Cell Lymphoma Recurrent | Drug: Ibrutinib-RICE | Singapore General Hospital|Janssen, LP | Phase 2 | 2016-12-13 | 2017-02-13 |
| NCT02801578 | Chronic Lymphocytic Leukemia | Drug: Ibrutinib | M.D. Anderson Cancer Center |
| 2016-07-01 | 2016-10-27 |
| NCT02757040 | Leukemia, Lymphocytic, Chronic, B-Cell | Drug: Ibrutinib combined with As2O3|Drug: ibrutinib | Peking University People's Hospital|Beijing Hospital | Phase 3 | 2016-12-01 | 2016-04-27 |
| NCT02973399 | Cancer | Drug: SNX-5422 plus ibrutinib | Esanex Inc. | Phase 1 | 2017-02-07 | 2017-02-13 |
| NCT02912754 | Leukemia, Lymphocytic, Chronic, B-Cell | Drug: ruxolitinib|Drug: ibrutinib | Sunnybrook Health Sciences Centre|Novartis | Phase 1|Phase 2 | 2017-03-01 | 2017-03-08 |
| NCT02884453 | Gastrooesophageal Cancer | Drug: ibrutinib | Royal Marsden NHS Foundation Trust|Janssen, LP | Phase 2 | 2016-07-01 | 2016-08-25 |
| NCT02914327 | Cancer | Drug: SNX-5422 plus ibrutinib | Esanex Inc. | Phase 1 | 2017-02-02 | 2017-02-13 |
| NCT02943473 | High Risk Smoldering Multiple Myeloma | Drug: Ibrutinib | Icahn School of Medicine at Mount Sinai|Pharmacyclics LLC. | Phase 2 | 2016-11-01 | 2016-11-15 |
| NCT02315326 | Refractory/Recurrent Primary Central Nervous System Lymphoma (PCNSL)|Refractory/Recurrent Secondary Central Nervous System Lymphoma (SCNSL) | Drug: Ibrutinib|Drug: HD- Methotrexate (MTX)|Drug: Rituximab + HD- Methotrexate (MTX) | Memorial Sloan Kettering Cancer Center|Pharmacyclics LLC.|Janssen Biotech, Inc. | Phase 1|Phase 2 | 2014-12-01 | 2017-02-22 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们