-
生物活性
AC0010 is a pyrrolopyrimidine-based irreversible EGFR inhibitor, structurally distinct from previously reported pyrimidine-based irreversible EGFR inhibitors, such as osimertinib and rociletinib. AC0010 selectively inhibits EGFR-active and T790M mutations with up to 298-fold increase in potency compared with wild-type EGFR.
Inhibitoryactivity of AC0010[1]
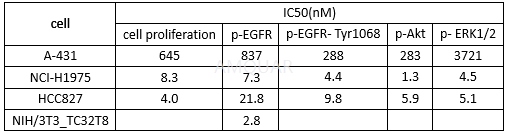
Kinaseactivities of AC0010[1]
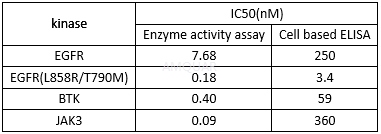
Anti-proliferationof AC0010[1]
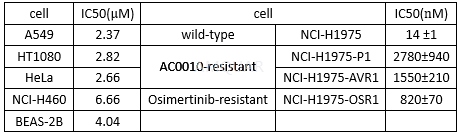
-
体外研究
-
体内研究
-
激酶实验
In vitro kinase activity assay[1]
For the single dose screening assay, AC0010concentration at either 1 µM or 10 µM was used. For IC50 value determinations,a 10-concentration gradient from 5.1x10-11-1.0x10-6 mol/Lwas set for the tested compounds. Staurosporine served as a control compound tomonitor assay quality for IC50 value determinations and one-dose kinaseactivity assay.
-
细胞实验
Cell culture[1]
The NCI-H1975, HCC827, A431, A549,NCI-H460, HT1080, HeLa, BEAS-2B, and NIH/3T3 cells were maintained at 37oCwith 5% CO2 in the media supplemented with 10% FBS, penicillin (100U/mL), and streptomycin (100 mg/mL). Cells were routinely screened formycoplasma and periodically authenticated by morphologic inspections.
Engineeredcell lines and resistant cell lines
NIH/3T3 cells harboring EGFR L858R/T790Mdouble mutations were engineered by exposing NIH/3T3 cells to the lentivirus thatexpressed human EGFR (L858R/T790M), and then followed by clone selection usingpuromycin. The resistant colonies were selected and expanded for furthersequencing analysis to confirm the integration of EGFR L858R/T790M mutations. Aconfirmed clone was subsequently established to be a cell line designated asNIH/3T3_TC32T8.
Resistant cell line NCI-H1975-P1 wasderived from relapsed tumor tissues of NCI-H1975 xenograft mouse that developedresistance to AC0010. AC0010-resistant NCI-H1975-AVR1 cells andosimertinib-resistant NCI-H1975-OSR1 cells were generated in vitro by exposingNCI-1975 cells to escalating doses of AC0010 and osimertinib, respectively, fora prolonged period.
Cellproliferation assays
Cell proliferation was assayed by a cellviability reagent, WST-1, per instruction from the manufacturer. Cells wereseeded at optimal density onto 96-well plates and incubated for 24 hours,followed by compound treatment for 72 hours. Cell viability was then assayed byincubating cells with WST-1 reagent for 2 to 3 hours.
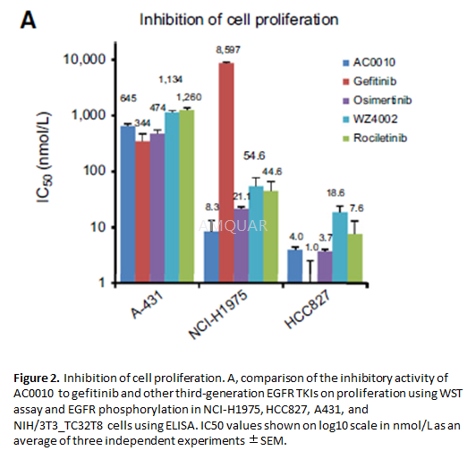
-
动物实验
Xenograft models[1]
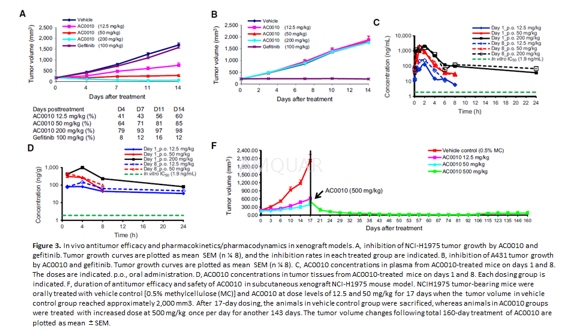
The Nu/Nu nude mice were used during the experiment.Six- to 8-week-old female mice were inoculated subcutaneously at the rightflank with approximately 3–5 x 106 cells in 0.2 mL of medium fortumor development. The treatments were started when the tumor size reachedapproximately 200 mm3. For NCI-H1975 and A431 models, mice were dividedinto five groups (n = 8/group), including a vehicle group (0.5%methylcellulose), three AC0010-testing groups treated with AC0010MA at thedoses of 12.5, 50, and 500 mg/kg, and a control group treated with gefitinib at100 mg/kg. All mice were orally administrated once daily for 14 consecutivedays.
For the long-term treatment study,NCI-H1975 tumor-bearing mice with tumor volume of 170 mm3 wereorally treated with a vehicle control (0.5% methylcellulose), AC0010 at dose levelsof 12.5 and 50 mg/kg for 17 days when the tumor volume in vehicle control groupreached approximately 2,000 mm3. After 17-day dosing, animals in thevehicle control group were sacrificed, whereas animals in AC0010 groups werecontinually daily administrated with increased dose at 500 mg/kg till the testmice could not tolerate the treatment. Mouse body weight and tumor volume weremeasured twice per week. Tumor volume was then used for the calculation oftumor inhibitory rate and tumor regression rate.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Xu X, Mao L, Xu W, et al. AC0010, an Irreversible EGFR Inhibitor Selectively Targeting Mutated EGFR and Overcoming T790M-Induced Resistance in Animal Models and Lung Cancer Patients. Mol Cancer Ther. 2016;15(11):2586-2597.
[2] Ivana Sullivan, David Planchard. Next-Generation EGFR Tyrosine Kinase Inhibitors for Treating EGFR-Mutant Lung Cancer beyond First Line. Front Med (Lausanne) 2016; 3: 76. Published online 2017 Jan 18. doi: 10.3389/fmed.2016.00076.
[3] Xu. Parallel phase 1 clinical trials in the US and in China: accelerating the test of avitinib in lung cancer as a novel inhibitor selectively targeting mutated EGFR and overcoming T790Minduced resistance. Chin J Cancer. 2015; 34: 27. Published online 2015 Jul 8. doi: 10.1186/s40880-015-0029-3.
分子式
C26H26FN7O2 |
分子量
487.53 |
CAS号
1557267-42-1 |
储存方式
﹣20 ℃冷藏长期储存。冰袋运输 |
溶剂(常温)
|
DMSO
|
Water
<1 mg/mL |
Ethanol
<1 mg/mL |
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT02274337 | Non-Small Cell Lung Cancer | Drug: AC0010 | Sun Yat-sen University|Acea Bio (Hangzhou) Co., Ltd.|Hangzhou ACEA Pharmaceutical Research Co.,Ltd. | Phase 1|Phase 2 | 2014-09-01 | 2016-11-21 |
| NCT02330367 | Metastatic Non-small Cell Lung Cancer | Drug: AC0010 | Hangzhou ACEA Pharmaceutical Research Co.,Ltd.|Guangdong General Hospital|Acea Bio (Hangzhou) Co., Ltd. | Phase 1 | 2015-01-01 | 2017-03-06 |
| NCT03060850 | B-cell Lymphoma | Drug: AC0010MA | Hangzhou ACEA Pharmaceutical Research Co.,Ltd. | Phase 1 | 2017-02-28 | 2017-03-06 |
| NCT02448251 | Non Small Cell Lung Cancer | Drug: AC0010MA | ACEA Biosciences, Inc. | Phase 1|Phase 2 | 2015-05-01 | 2016-07-26 |
| NCT03053219 | Carcinomaon-Small-Cell Lung | Drug: AC0010 | Hangzhou ACEA Pharmaceutical Research Co.,Ltd. | Phase 1 | 2016-11-01 | 2017-02-10 |
| NCT03001609 | Carcinoma, Non-Small-Cell Lung | Drug: 14C-labeled AC0010 oral | Hangzhou ACEA Pharmaceutical Research Co.,Ltd. | Phase 1 | 2016-11-01 | 2016-12-20 |
| NCT03058094 | NSCLC | Drug: Pemetrexed|Drug: Cisplatin 75mg/m2|Drug: AC0010 | Hangzhou ACEA Pharmaceutical Research Co.,Ltd. | Phase 3 | 2017-04-01 | 2017-02-15 |
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们





















