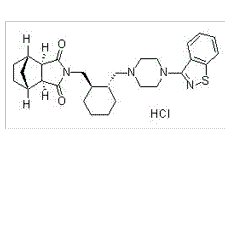
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00549718 | Schizophrenia | Drug: Lurasidone HCl | Sunovion | Phase 3 | 2007-10-01 | 2014-06-05 |
| NCT01074073 | Schizophrenia | Drug: Lurasidone HCl | Sunovion | Phase 1 | 2008-08-01 | 2011-09-06 |
| NCT01082263 | Schizophrenia Patients | Drug: Lurasidone HCl | Sunovion | Phase 1 | 2008-10-01 | 2011-09-06 |
| NCT01082276 | Male, Healthy Normal Subjects | Drug: Lurasidone HCl | Sunovion | Phase 1 | 2008-08-01 | 2011-09-06 |
| NCT01074632 | Schizophrenia, Schizoaffective Disorder, or Schizophreniform Disorder | Drug: Lurasidone HCl | Sunovion | Phase 1 | 2009-05-01 | 2011-09-06 |
| NCT00711269 | Schizophrenia | Drug: SM-13496 (lurasidone HCl)|Drug: SM-13496 (lurasidone HCl)|Drug: Placebo|Drug: Risperidone | Sumitomo Dainippon Pharma Co., Ltd. | Phase 3 | 2008-07-01 | 2012-06-11 |
| NCT00615433 | Schizophrenia | Drug: Lurasidone|Drug: Olanzapine|Drug: Placebo comparator|Drug: Lurasidone 40 mg tablets | Sunovion | Phase 3 | 2008-01-01 | 2015-05-19 |
| NCT00641745 | Schizophrenia|Schizoaffective Disorder | Drug: Lurasidone HCl|Drug: Risperidone | Sunovion | Phase 3 | 2008-03-01 | 2015-06-16 |
| NCT00549666 | Pharmacokinetics | Drug: Lurasidone 40 mg|Drug: Placebo 40 mg|Drug: Ortho Tri-Cyclen | Sunovion | Phase 1 | 2007-08-01 | 2011-09-12 |
| NCT01614912 | Schizophrenia | Drug: SM-13496 | Sumitomo Dainippon Pharma Co., Ltd. | Phase 3 | 2012-08-01 | 2015-06-30 |
| NCT01614899 | Schizophrenia | Drug: SM-13496 40mg|Drug: SM-13496 80mg|Drug: Placebo | Sumitomo Dainippon Pharma Co., Ltd. | Phase 3 | 2012-06-01 | 2015-06-30 |
| NCT01082250 | Schizophrenia | Drug: Lurasidone HCl | Sunovion | Phase 1 | 2008-07-01 | 2011-09-06 |
| NCT01143090 | Schizophrenia|Schizoaffective Disorder | Drug: Lurasidone HCl | Sunovion | Phase 3 | 2010-08-01 | 2015-05-19 |
| NCT01143077 | Schizophrenia|Schizoaffective Disorder | Drug: Lurasidone HCl | Sunovion | Phase 3 | 2010-06-01 | 2013-04-09 |
| NCT00789698 | Chronic Schizophrenia | Drug: Lurasidone HC1|Drug: Quetiapine XR | Sunovion | Phase 3 | 2008-12-01 | 2015-05-19 |
| NCT00790192 | Schizophrenia | Drug: Lurasidone|Drug: Lurasidone|Drug: Quetiapine XR|Drug: Placebo | Sunovion | Phase 3 | 2008-10-01 | 2016-03-11 |
| NCT01082289 | Schizophrenia | Drug: Lurasidone | Sunovion | Phase 1 | 2008-09-01 | 2011-09-06 |
| NCT01986114 | Bipolar I Disorder | Drug: SM-13496 (lurasidone HCl) | Sumitomo Dainippon Pharma Co., Ltd. | Phase 3 | 2013-11-01 | 2017-02-24 |
| NCT01986101 | Bipolar Depression | Drug: SM-13496 (lurasidone HCl)|Drug: Placebo | Sumitomo Dainippon Pharma Co., Ltd. | Phase 3 | 2013-11-01 | 2017-02-24 |
| NCT01421134 | Major Depressive Disorder With Mixed Features | Drug: Lurasidone|Drug: Placebo | Sunovion | Phase 3 | 2011-09-01 | 2016-07-13 |