- Home
-
Signal Pathway
- 代理抗体
- 仪器
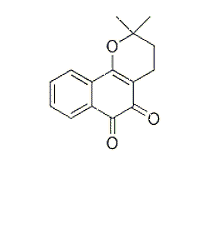
- AMQUAR单克隆抗体抑制剂
- Apoptosis
- Angiogenesis
- Autophagy
- Cell Cycle
- Cytoskeletal Signalling
- DNA Damage/DNA Repair
- Endocrinology & Hormones
- Epigenetics
- GPCR & G Protein
- 5-HT Receptor
- Adenosine Receptor
- Adrenergic Receptor
- Angiotensin Receptor
- Bombesin Receptors
- Cannabinoid Receptor
- CaSR
- Cholecystokinin (CCK)
- CCR
- CGRP Receptor
- CRF Receptors
- CXCR
- Dopamine Receptor
- Endothelin Receptor
- GNRH Receptor
- GHSR
- GPR109A
- GPR119
- GPR120(FFA4)
- GPR35
- GPR40(FFAR1)
- GPR55
- GPR84
- Glucagon Receptor
- Glucocorticoid Receptor
- Histamine Receptor
- Leukotriene Receptor
- LPA Receptor
- LPL Receptor
- mAChR
- Melatonin Receptor
- mGluR
- Neurokinin Receptor
- Opioid Receptor
- Orexin Receptor(OX Receptor)
- PARs
- PACAP
- P2Y Receptor
- PAF-R
- Prostaglandin Receptor
- Ras
- RGS
- S1P Receptor
- SGLT
- Sigma Receptor
- Somatostatin Receptor
- TSH Receptor
- Urotensin-II Receptor
- Vasopressin Receptor
- JAK/STAT
- Microbiology
- Metabolism
- 15-PGDH
- 5-alpha Reductase
- 5-Lipoxygenase
- Adenosine Deaminase
- AhR
- Aminopeptidase
- Carbonic Anhydrase
- CETP
- CPT1
- Cytochromes P450 (CYPs)
- Dehydrogenase
- DGAT
- DHFR
- Dopamine β-hydroxylase
- FAAH
- Factor Xa
- Glucokinase
- elastase
- HMG-CoA Reductase (HMGCR)
- HSP
- IDO
- MAO
- NAMPT
- PPAR
- Procollagen C Proteinase
- RARs/RXR
- SCD
- SGLT
- TPH
- Transferase
- Phosphodiesterase (PDE)
- Ferroptosis
- Phospholipase
- MAPK
- Neuronal Signalling
- NF-κB
- Protein Tyrosine Kinase
- Proteases
- PI3K/Akt/mTOR
- Stem Cells & Wnt
- Transmembrane Transporters
- TGF-beta/Smad
- Ubiquitin
- Others
- 生物试剂
- 高通量筛选分子库
- Tech Support
- Service
- News Center
- Contact Us