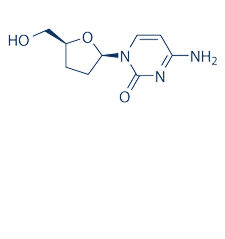
| NCT Number | Conditions | Interventions | Sponsor/Collaborators | Phases | Start Date | Last Updated |
| NCT00002118 | HIV Infections | Drug: Zalcitabine | Hoffmann-La Roche|NIH AIDS Clinical Trials Information Service | | | 2005-06-23 |
| NCT00002256 | HIV Infections | Drug: Zalcitabine | Hoffmann-La Roche|NIH AIDS Clinical Trials Information Service | | | 2005-06-23 |
| NCT00002333 | HIV Infections | Drug: Saquinavir|Drug: Zalcitabine | Hoffmann-La Roche|NIH AIDS Clinical Trials Information Service | Phase 2 | | 2005-06-23 |
| NCT00000653 | HIV Infections | Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche | Phase 2 | | 2012-03-29 |
| NCT00000997 | HIV Infections | Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID) | | | 2012-03-15 |
| NCT00000704 | HIV Infections | Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID) | Phase 1 | | 2012-03-15 |
| NCT00002265 | HIV Infections | Drug: Zalcitabine | Hoffmann-La Roche|NIH AIDS Clinical Trials Information Service | Phase 2 | | 2005-06-23 |
| NCT00000719 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID) | | | 2012-03-28 |
| NCT00001040 | HIV Infections | Drug: Saquinavir|Drug: Zidovudine|Drug: Zalcitabine | Hoffmann-La Roche|National Institute of Allergy and Infectious Diseases (NIAID) | Phase 2 | | 2011-02-28 |
| NCT00002081 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | Hoffmann-La Roche|NIH AIDS Clinical Trials Information Service | | | 2005-06-23 |
| NCT00000651 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche|Glaxo Wellcome | Phase 3 | | 2012-03-28 |
| NCT00000679 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche | Phase 2 | | 2011-03-11 |
| NCT00002117 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | Hoffmann-La Roche|NIH AIDS Clinical Trials Information Service | Phase 3 | | 2005-06-23 |
| NCT00000678 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche | Phase 2 | | 2011-03-11 |
| NCT00000978 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche | Phase 1 | | 2008-08-06 |
| NCT00000969 | HIV Infections | Drug: Zalcitabine|Drug: Didanosine | National Institute of Allergy and Infectious Diseases (NIAID)|Bristol-Myers Squibb|Hoffmann-La Roche | 2013-09-28 |
| NCT00001032 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID) | Phase 2 | | 2012-03-29 |
| NCT00000718 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine | National Institute of Allergy and Infectious Diseases (NIAID)|Hoffmann-La Roche | | 2012-03-28 |
| NCT00002279 | HIV Infections | Drug: Zalcitabine | Hoffmann-La Roche|NIH AIDS Clinical Trials Information Service | | | 2005-06-23 |
| NCT00002086 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine|Drug: Interferon alfa-n1 | Glaxo Wellcome|NIH AIDS Clinical Trials Information Service | Phase 2 | | 2005-06-23 |
| NCT00001022 | HIV Infections | Drug: Zidovudine|Drug: Zalcitabine|Drug: Didanosine | National Institute of Allergy and Infectious Diseases (NIAID) | Phase 3 | | 2013-09-28 |