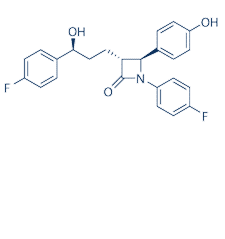
| NCT00762229 | Hypercholesterolemia | Drug: Ezetimibe 10 mg|Drug: Ezetimibe 5 mg | Bronx VA Medical Center | | 2007-07-01 | 2013-06-12 |
| NCT00794677 | Hypercholesterolemia | Drug: ezetimibe | Children's Hospital & Research Center Oakland|Merck Schering-Plough | Phase 4 | 2006-06-01 | 2009-07-31 |
| NCT00651274 | Atherosclerosis|Hypercholesterolemia|Coronary Heart Disease | Drug: Ezetimibe|Drug: Placebo | Merck Sharp & Dohme Corp. | Phase 4 | 2003-04-01 | 2015-04-15 |
| NCT00634140 | Chronic Acalculous Cholecystitis | Drug: ezetimibe|Drug: Placebo | Indiana University | | 2009-08-01 | 2015-03-27 |
| NCT00202904 | Hypercholesterolemia|Coronary Heart Disease | Drug: ezetimibe|Drug: placebo | Merck Sharp & Dohme Corp. | Phase 4 | 2005-05-01 | 2015-04-28 |
| NCT00621699 | Pharmacokinetics|Drug Interactions|Hypercholesterolemia|Immunosuppression | Drug: 1 tablet Ezetrol(R) (ezetimibe), MSD Sharp & Dohme GmbH, Germany|Drug: 1 capsule Prograf(R) (tacrolimus), Astellas Pharma GmbH, Germany|Drug: 1 tablet Ezetrol(R) + 1 capsules Prograf(R) | University Medicine Greifswald | Phase 1 | 2007-09-01 | 2008-02-12 |
| NCT00621101 | Pharmacokinetics|Drug Interactions|Hypercholesterolemia|Immunosuppression | Drug: 1 tablet Ezetrol(R) (ezetimibe), MSD Sharp & Dohme GmbH, Germany|Drug: 1 tablet Rapamune(R) (sirolimus), Wyeth Pharma, Germany|Drug: 1 tablet Ezetrol(R) + 1 tablet Rapamune(R) | University Medicine Greifswald | Phase 1 | 2007-04-01 | 2008-02-12 |
| NCT00653523 | Hypercholesterolemia | Drug: Ezetimibe|Drug: Simvastatin | Merck Sharp & Dohme Corp. | Phase 3 | 2007-12-01 | 2015-06-09 |
| NCT00654095 | Hypercholesterolemia | Drug: Ezetimibe|Drug: atorvastatin | Merck Sharp & Dohme Corp. | Phase 3 | 2007-12-01 | 2015-06-09 |
| NCT00653445 | Hypercholesterolemia|Coronary Heart Disease|Atherosclerosis | Drug: Rosuvastatin|Drug: Ezetimibe | AstraZeneca | Phase 3 | 2004-06-01 | 2009-03-25 |
| NCT00704535 | Primary Hypercholesterolemia|Homozygous Familial Hypercholesterolemia | Drug: Ezetimibe | Merck Sharp & Dohme Corp. | | 2006-03-01 | 2015-04-09 |
| NCT02029625 | Dyslipidemia | Drug: NVP-1205|Drug: rosuvastatin and ezetimibe | Navipharm Corporation | Phase 1 | 2014-02-01 | 2016-03-24 |
| NCT00908011 | Hypercholesterolemia|HIV Infections | Drug: Ezetimibe|Drug: Rosuvastatin (standard care) | University of British Columbia|Merck Frosst-Schering Pharma, G.P. | | 2009-06-01 | 2015-11-30 |
| NCT00738985 | Cardiovascular Diseases|Hyperlipidemia | Drug: ezetimibe/simvastatin 10/20 mg + placebo|Drug: ezetimibe/simvastatin 10/20 mg + MK0524A | Instituto Nacional de Ciencias Medicas y Nutricion Salvador Zubiran|Merck Sharp & Dohme Corp. | Phase 4 | 2009-11-01 | 2015-03-23 |
| NCT00867165 | Primary Hypercholesterolemia | Drug: ezetimibe|Drug: Placebo | Merck Sharp & Dohme Corp. | Phase 3 | 2009-05-01 | 2015-04-20 |
| NCT02548936 | Atherosclerosis | Drug: Ezetimibe+Simvastatin Drug Combination | Peking Union Medical College Hospital | Early Phase 1 | 2015-04-01 | 2015-09-11 |
| NCT00319449 | Hypercholesterolemia|Coronary Arteriosclerosis | Drug: Ezetimibe|Drug: Placebo|Drug: Atorvastatin 10 mg | Merck Sharp & Dohme Corp.|PT. Schering-Plough. Tbk Indonesia | Phase 4 | 2005-09-01 | 2015-10-09 |
| NCT01384058 | Diabetes Mellitus Type 2|Hypercholesterolemia | Drug: ezetimibe|Drug: simvastatin|Drug: Ezetimibe 10/Simvastatin 20 | University Hospital Freiburg|Essex Pharma GmbH | Phase 4 | 2007-11-01 | 2012-06-18 |
| NCT00653913 | Hypercholesterolemia | Drug: SCH 58235|Drug: pitavastatin | Merck Sharp & Dohme Corp. | Phase 1 | 2004-03-01 | 2015-04-07 |
| NCT02682680 | Type 2 Diabetes Mellitus | Drug: Colesevelam|Drug: Ezetimibe | LMC Diabetes & Endocrinology Ltd.|Valeant Pharmaceuticals International, Inc. | Phase 4 | 2016-01-01 | 2016-02-10 |
| NCT01008345 | Coronary Artery Disease|Diabetes | Drug: ezetimibe | Hotel Dieu de France Hospital|Pharmaline, Lebanon | Phase 4 | 2008-09-01 | 2009-11-05 |