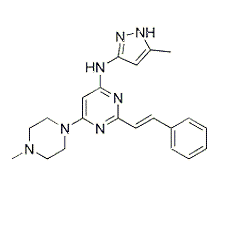
| NCT01914510 | Ovarian Clear Cell Carcinoma | Drug: ENMD-2076 | University Health Network, Toronto | Phase 2 | 2013-09-01 | 2016-08-05 |
| NCT01719744 | Advanced|Metastatic|Soft Tissue Sarcoma | Drug: ENMD-2076 | University Health Network, Toronto | Phase 2 | 2013-01-01 | 2016-03-14 |
| NCT01639248 | Triple Negative Breast Cancer | Drug: ENMD-2076 | CASI Pharmaceuticals, Inc.|University of Colorado, Denver|Indiana University Melvin and Bren Simon Cancer Center | Phase 2 | 2012-07-01 | 2016-07-25 |
| NCT02234986 | Advanced Adult Hepatocellular Carcinoma|Advanced Fibrolamellar Carcinoma | Drug: ENMD-2076 | CASI Pharmaceuticals, Inc. | Phase 2 | 2015-10-01 | 2016-03-19 |
| NCT00904787 | Relapsed or Refractory Hematological Malignancies | Drug: ENMD-2076 | CASI Pharmaceuticals, Inc. | Phase 1 | 2009-04-01 | 2011-08-03 |
| NCT00806065 | Multiple Myeloma | Drug: ENMD-2076 | CASI Pharmaceuticals, Inc. | Phase 1 | 2008-12-01 | 2012-01-19 |
| NCT00658671 | Advanced Cancer | Drug: ENMD-2076 | CASI Pharmaceuticals, Inc. | Phase 1 | 2008-04-01 | 2016-01-26 |
| NCT01104675 | Ovarian Cancer|Fallopian Cancer|Peritoneal Cancer | Drug: ENMD-2076 | CASI Pharmaceuticals, Inc. | Phase 2 | 2010-04-01 | 2014-08-04 |