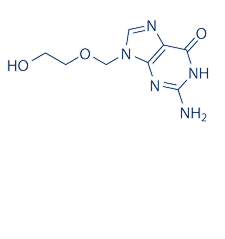
| NCT00495573 | Genital Herpes | Drug: acyclovir | University of Washington|National Institutes of Health (NIH) | | 2007-06-01 | 2012-03-21 |
| NCT00031447 | Herpes Simplex | Drug: Acyclovir|Drug: Placebo | National Institute of Allergy and Infectious Diseases (NIAID) | Phase 3 | 1999-08-01 | 2012-05-10 |
| NCT00031460 | Herpes Simplex | Drug: Acyclovir|Drug: Placebo | National Institute of Allergy and Infectious Diseases (NIAID) | Phase 3 | 1997-12-01 | 2012-05-10 |
| NCT00723229 | Genital Herpes | Drug: acyclovir | University of Washington|National Institutes of Health (NIH) | Phase 4 | 2008-08-01 | 2017-03-06 |
| NCT00371592 | HIV Infections|Herpesvirus 2, Human | Drug: Acyclovir|Drug: Acyclovir placebo | National Institute of Allergy and Infectious Diseases (NIAID)|Comprehensive International Program of Research on AIDS | Phase 2 | 2006-09-01 | 2013-09-20 |
| NCT00209313 | HIV-1 and HSV-2 Coinfection|HIV Infections | Drug: Acyclovir | Fred Hutchinson Cancer Research Center|Institute for the Development of Africa | Phase 2|Phase 3 | 2005-03-01 | 2011-12-14 |
| NCT02053142 | Ulcers of Female Genital Organs | Drug: Acyclovir|Drug: Placebo | University of North Carolina, Chapel Hill|National Institute of Allergy and Infectious Diseases (NIAID) | | 2009-01-01 | 2014-12-11 |
| NCT00076232 | HIV Infections|HIV Seronegativity|Herpes Genitalis | Drug: Acyclovir|Drug: Acyclovir placebo | National Institute of Allergy and Infectious Diseases (NIAID)|Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)|National Institute on Drug Abuse (NIDA)|National Institute of Mental Health (NIMH) | Phase 3 | 2005-04-01 | 2010-12-29 |
| NCT00942084 | Herpes Simplex Virus|Neonatal Sepsis | Drug: Acyclovir | Phillip Brian Smith|Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD)|Duke University | Phase 1 | 2011-09-01 | 2013-11-19 |
| NCT01327144 | Herpes Zoster | Drug: Famciclovir|Drug: Aciclovir | EMS | Phase 3 | 2012-06-01 | 2017-02-03 |
| NCT01714180 | Hematological Malignancy|Pharmacokinetics of Acyclovir | | West Virginia University | | 2012-10-01 | 2015-06-02 |
| NCT00711776 | Herpes Labialis | Drug: New formulation|Drug: Current formulation | GlaxoSmithKline | Phase 1 | 2008-04-01 | 2013-03-21 |
| NCT00362297 | Genital Herpes | Drug: acyclovir|Drug: valacyclovir | University of Washington | Phase 4 | 2006-09-01 | 2014-10-02 |
| NCT01026454 | HSV Infection|HIV Infection | Drug: acyclovir|Drug: valacyclovir | University of Washington | Phase 4 | 2010-02-01 | 2014-03-10 |
| NCT01729767 | Meniere's Disease | Drug: Acyclovir|Drug: Placebo | Tehran University of Medical Sciences | Phase 2|Phase 3 | 2011-08-01 | 2012-11-19 |
| NCT00855309 | Herpes Simplex | Drug: acyclovir sodium | Wake Forest University Health Sciences|National Cancer Institute (NCI) | Phase 3 | 2008-11-01 | 2017-01-17 |
| NCT02255734 | Healthy | Drug: Acyclovir | Yung Shin Pharm. Ind. Co., Ltd. | Phase 4 | 2014-04-01 | 2014-09-29 |
| NCT00405821 | HIV Infections|Herpes Genitalis | Drug: Acyclovir|Drug: Placebo | National Institute of Allergy and Infectious Diseases (NIAID)|University of Washington|Johns Hopkins University|Translational Genomics Research Institute|National Institutes of Health Clinical Center (CC) | Phase 2 | 2006-11-01 | 2012-08-28 |
| NCT00362596 | HIV|Herpes | Drug: acyclovir | Centers for Disease Control and Prevention|Ministry of Health, Thailand | Phase 4 | 2005-01-01 | 2006-08-09 |
| NCT00808405 | Genital Herpes | Drug: acyclovir|Drug: matching placebo | University of Washington|National Institute of Allergy and Infectious Diseases (NIAID)|National Institutes of Health (NIH) | | 2009-01-01 | 2013-11-15 |
| NCT01281007 | GENITAL HERPES | Drug: Famciclovir|Drug: Aciclovir | EMS | Phase 3 | 2012-07-01 | 2015-07-03 |