-
生物活性
Thebinding affinities (Kd) of pembrolizumab to hPD-1 is 27.0 pmol/L.[2]
-
体外研究
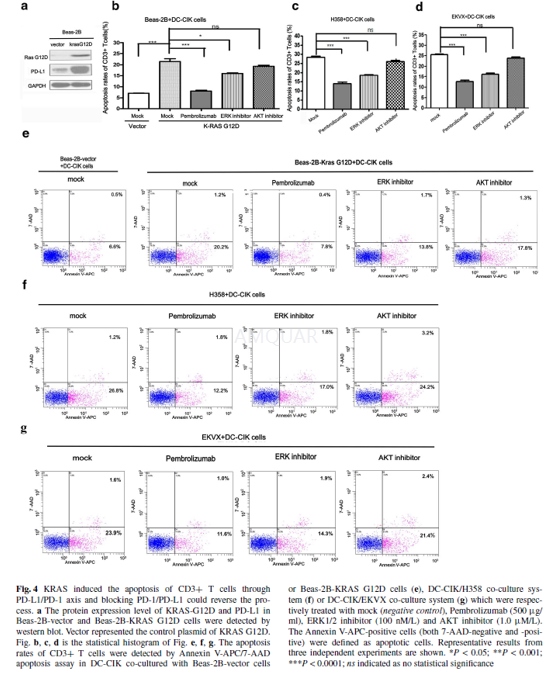
Pembrolizumab could re-activate the anti-tumor immunity of T cells and decrease the survivalrates of NSCLC cells with endogenous KRAS mutation in co-culture system. Blockadeof PD-1 on T cells with Pembrolizumab in Beas-2B-KRAS G12D/DC-CIK co-culturesystem reduced the apoptosis rates of CD3+ T cells.[1]
-
体内研究
-
激酶实验
Receptor Occupancy Measurement[3]
Single-cell suspensions of tumor and bloodcollected from mice treated with rat DX400 were split into saturation anddetection samples. Samples were incubated with mouse IgG to block Fc receptors.The saturation sample was incubated with a saturating concentration for the ratDX400. All samples were incubated with a phycoerythrinlabeled anti-rat IgGantibody. Flow-cytometry analysis was used to measure fluorescence in both the saturationand detection samples. Receptor occupancy was calculated as the ratio of fluorescencein the detection and saturation samples.
-
细胞实验
Cell lines and cell culture[1]
Human NSCLC cell lines H460, H1299, H2228,H292 and H1993, immortalized human lung bronchial epithelial cell (Beas-2B),EKVX, Beas-2B-vector, Beas-2B-KRASG12D and Beas-2B-KRAS-WT cells were prepared.H2228 and H358 were cultured in RPMI-1640 complete growth medium supplementedwith 10% fetal bovine serum and antibiotics (10,000U/ml penicillin and 10μg/mlstreptomycin). Other cell lines were grown in DMEM complete medium.
Co-culturesystem and apoptosis assay with flow cytometry
Beas-2B-KRASG12D, Beas-2B-vector, H358 andEKVX cells were seeded into 12-well plates at a density of 1.0 × 105 cells/ well, respectively. The acquired DC-CIK were added into co-culture systemwith Beas-2B-KRAS-G12D, Beas-2Bvector, H358 or EKVX cells at the ratio of 1:1,respectively. Next, the DC-CIK/Beas-2B-KRAS-G12D, DC-CIK/ H358 and DC-CIK/EKVXco-culture system were treated with mock, Pembrolizumab (500μg/ml), ERK1/2inhibitor (100 nM/L) or AKT inhibitor (1.0 μM/L), respectively. After 48 h,suspended DC/CIK cells were removed from the adherent Beas-2B-KRAS-G12D, H358or EKVX cells in the cell culture plate with pipet. Then, after washing withPBS, suspended DC/CIK cells were stained with anti-human CD3 FITC antibody(OKT-3, 11-0037) for 15 min. Next, after washing, DCCIK cells were stained withAnnexin V-APC and 7-AAD for 15 min with Apoptosis Detection kit (KGA1023-1026).The apoptotic cells of CD3+ T cells detected by flow cytometry were defined asAnnexin V-APC-positive cells (both 7-AAD-negative and -positive) from the gateof CD3-positive cells.
Realtime cells survival analysis
The survival rates of KRAS-mutant tumorcells like H358 or EKVX cells were dynamically monitored in real time by thexCELLigence system which could exclude the interference of suspended DC-CIK.Firstly96-well E-plate with 50μl of complete growth medium in each well wastested in the incubator to establish a background reading. Next, tumor cells(1.0 × 104 cells/well) were seeded into 96-well E-plates forapproximately 20 h followed by addition of DC-CIK (50μl/well) into the E-platesat a DC-CIK: tumor cells ratio of 1:1. Finally, an additional 50 μl/well of thecomplete medium containing different drugs such as vehicle, Pembrolizumab (500μg/ ml), ERK1/2 inhibitor (100 nM/L) and Pembrolizumab (500 μg/ml) plus ERK1/2inhibitor (100 nM/L) were added into the DC-CIK/H358 or DC-CIK/EKVX co-culturesystem, respectively. H358 cells alone were meanwhile treated with vehicle,Pembrolizumab (500 μg/ml) and ERK1/2 inhibitor (100 nM/L) as the controlgroups. Cell index values were monitored every 15 min from each well of E-plateand presented as the dynamic cell growth curves.

-
动物实验
Mouse PK and PK/PD model development[3]
Syngeneic (i.e., allograft) C57BL/6 micewith intact immune systems were used in the preclinicalexperiments. Sincepembrolizumab does not crossreact with rodent PD-1, mice received either achimeric mouse or parental rat DX400 anti–PD-1 antibody. Dose levels rangedfrom 0.1 to 10 mg/kg administered intravenously on days 0, 7, and 14, withfrequent blood sampling on days 0 and 4 with sparse sampling (Table S1). Plasmasamples were analyzed for mouse or rat DX400 concentration using anenzyme-linked immunosorbent assay. The minimum detectable concentration was0.312 μg/mL for mouse DX400 and 0.100μg/mL for ratDX400.
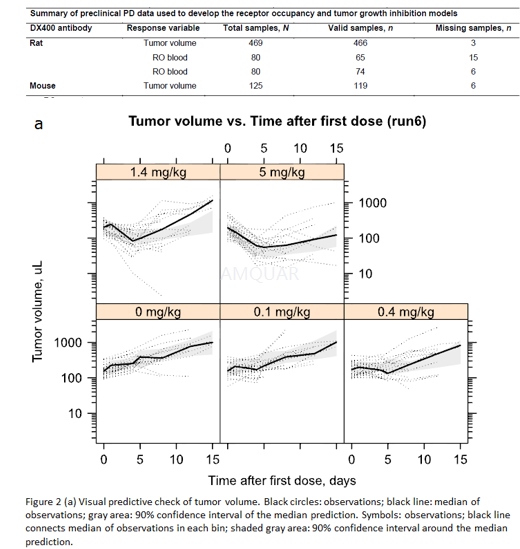
Tumor volume was assessed in MC38-bearingmice receiving vehicle or 0.1, 0.4, 1.4, or 5 mg/kg of rat DX400 on days 0 and4. Two perpendicular tumor diameters were measured at baseline and on days 1,4, 5, 8, 12, and 15 using digital calipers (0.01 mm accuracy). Tumor volume wascalculated using the ellipsoidal volume formula (0.5 x smallest diameter2 x largest diameter).
Qualification of the mouse PK/PD model wasachieved by performing external visual predictive checks (VPCs) using aseparate test dataset. Within this test dataset, individual tumor size datawere available from mice receiving vehicle or 0.1, 0.4, 1.4, or 5 mg/kg mouseDX400 on days 0, 5, 9, 13, and 17. Further details of the design are providedin Table S1. The mouse PK/PD model was used to simulate (with parameteruncertainty from bootstrap analysis) the time course of tumor volume under theexperimental design of the test dataset. Baseline tumor sizes between thestudies were adjusted for and VPCs were performed to compare simulated tumorvolumes (80% confidence interval [CI] around median prediction) to observedtumor volumes. The datasets for model building and testing used the rat andmouse DX400 antibodies, respectively. Given the lack of significant systematic differencesin PK between the two antibodies, the test dataset was considered appropriatefor the purpose of model qualification.


-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Chen, N.; Fang, W.; Lin, Z.; Peng, P.; Wang, J.; Zhan, J.; Hong, S.; Huang, J.; Liu, L.; Sheng, J.; Zhou, T.; Chen, Y.; Zhang, H.; Zhang, L., KRAS mutation-induced upregulation of PD-L1 mediates immune escape in human lung adenocarcinoma. Cancer Immunol Immunother 2017.
[2] Tan, S.; Chen, D.; Liu, K.; He, M.; Song, H.; Shi, Y.; Liu, J.; Zhang, C. W. H.; Qi, J.; Yan, J.; Gao, S.; Gao, G. F., Crystal clear: visualizing the intervention mechanism of the PD-1/PD-L1 interaction by two cancer therapeutic monoclonal antibodies. Protein & Cell 2016, 7 (12), 866-877.
[3] Lindauer, A.; Valiathan, C. R.; Mehta, K.; Sriram, V.; de Greef, R.; Elassaiss-Schaap, J.; de Alwis, D. P., Translational Pharmacokinetic/Pharmacodynamic Modeling of Tumor Growth Inhibition Supports Dose-Range Selection of the Anti-PD-1 Antibody Pembrolizumab. CPT Pharmacometrics Syst Pharmacol 2017, 6 (1), 11-20.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃保存,一年有效。 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们





















