-
生物活性
Tocilizumab is an anti-interleukin-6receptor (IL-6R) monoclonal antibody, currently approved for the treatment ofsystemic juvenile idiopathic arthritis (sJIA) and rheumatoid arthritis.
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
Cell culture[1]
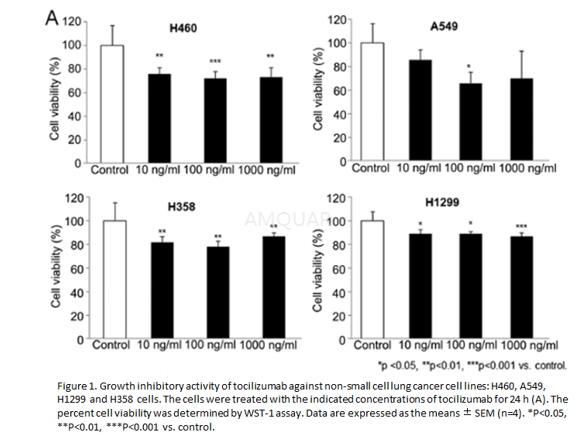
NSCLC cell lines H460, A549, H1299 and H358were grown in RPMI‑1640 medium with 10% heat‑inactivated fetal bovine serum and1% penicillin/streptomycin at 37˚C in a humidified incubator(5% CO2).
Cell proliferation assay
The anti‑proliferation activity oftocilizumab in NSCLC cells in vitro was measured using the EZ‑Cytox kit.Ten microliters of tocilizumab were added to 96‑well plates containing 104 cells per well in 100μl medium. The final concentrations of tocilizumab were 10, 100 and1000ng/ml. Following a 24‑h incubation, WST‑1 solution was added, and theoptical density was analyzed using the ELISA plate reader Magellan™ atreference wavelengths of 450 and 620 nm.
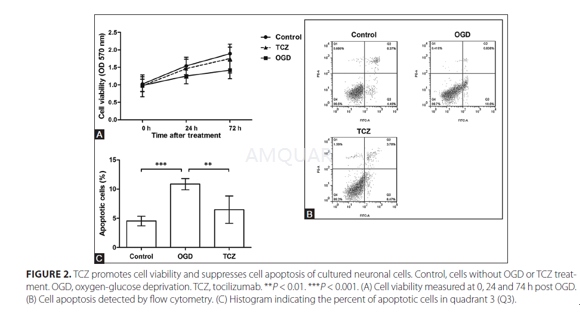
Cell culture and OGD[2]
Five newborn rats (1 d old) wereanesthetized and sacrificed for neuronal cell culture. Briefly, the hippocampalor cortical tissue was dissected and digested in 0.05% trypsin for 30 min.After centrifugation, the cell pellet was resuspended in Dulbecco’s modifiedeagle medium supplemented with 5% fetal bovine serum. Then the cells werecultured at a density of 1 × 106 cells/mL, and the medium wasreplaced by Neurobasal with B-27. The cells were divided into three groups,namely Control, oxygen-glucose deprivation (OGD) and tocilizumab (TCZ) group.For TCZ group, TCZ (10μg/mL) was added to the medium. After five days, OGD wasperformed. Briefly, the medium of OGD group was gradually replaced by glucose- freemedium and the final glucose concentration was lower than 1mM. Both OGD and TCZgroups were cultured in 95% N2 and 5% CO2, while Controlgroup continued to be cultured in atmosphere with 5% CO2. After 4 h,all the cells were cultured in the original conditions for further analysis.
Cellviability assay
After OGD, cells were adjusted to 2 × 104/mL,plated in 96-well plates, and cultured for another 0, 24 or 72 h, and collectedfor 3-(4,5-dimethylthiazol-2-yl)-2,5-dephenyltetrazolium bromide (MTT) assayusing MTT Cell Proliferation and Cytotoxicity Assay Kit according to themanuals. Briefly, 10μL MTT solution was added to each well and the cells werecultured for 4 h, after which 100μL Formanzan solution was added. The cellswere incubated until formanzan was dissolved. The optical density was measuredat 570 nm using a microplate reader.
Cellapoptosis assay
Cell apoptosis was analyzed using AnnexinV: FITC Apoptosis Detection Kit and a flow cytometer. Briefly, cells werecollected at 24 h post OGD, washed using cold phosphate buffer saline, andresuspended in binding buffer. Annexin V-FITC was added and the cells wereincubated in dark for 15 min. Before detection, prodium iodide (PI) and bindingbuffer were added. The percent of apoptotic cells were indicated as the AnenxinV-positive and PI-negative in quadrant 3.
-
动物实验
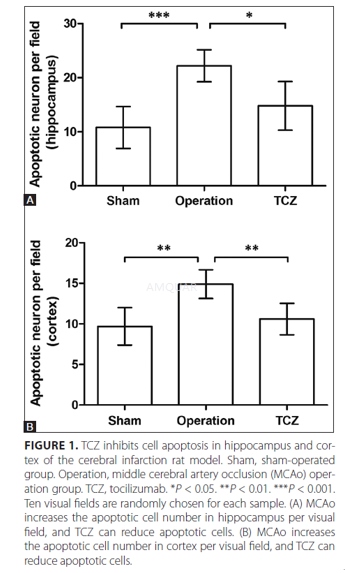
Animal model[2]
Male Sprague-Dawley rats of SPF degree(250-280 g) were randomly divided into three groups, namely Sham, Operation andTCZ groups, 15 individuals in each group. The animal experiments were approvedby a local animal ethics committee and our institution, and performed accordingto the rules of our laboratory.
Before the middle cerebral artery occlusion(MCAo) operation, rats of TCZ group were intraperitoneally injected with TCZ(8 mg/kg) daily for seven days. Then rats of Operation and TCZ groups underwentthe complete process of MACo. Briefly, 10% chloral hydrate wasintraperitoneally injected at 3.5 mL/kg for anesthetization. The skin wascleaned with iodine solution and a cervical midline incision was made. Thecommon carotid artery and external carotid artery were found and ligated with asuture, and the internal carotid artery was clipped with a vascular clamp. Thenan incision was made in the common carotid artery and a silicone-coated suturewas inserted, followed by a ligation. The reperfusion was performed after 2 h.After the rats were recovered from anesthetization, symptoms like standinginstability and left hemiparesis indicated the successful construction ofcerebral infarction model. Rats of Sham group underwent all the proceduresexcept the ligation. After the model was induced, rats of the three groups wereanesthetized and sacrificed for brain sampling. The hippocampus and cortexwere separately sampled for further analysis.
TUNELassay
The rat hippocampus and cortical wereparaffin-embedded, cut into slices, dewaxed and rehydrated. DNA fragmentation intissue cells was detected with the TdT-mediated dUTP nick-end labeling (TUNEL)method using In Situ Cell Death Detection Kit according to the manuals. Thetissue slices were incubated in TUNEL reaction mixture in dark for 60 min at37°C. Signals were observed using a fluorescence microscope. Ten visual fieldswere randomly selected for each sample to count the cells with positivesignals.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Kim, N. H.; Kim, S. K.; Kim, D. S.; Zhang, D.; Park, J. A.; Yi, H.; Kim, J. S.; Shin, H. C., Anti-proliferative action of IL-6R-targeted antibody tocilizumab for non-small cell lung cancer cells. Oncol Lett 2015, 9 (5), 2283-2288.
[2] Wang, S.; Zhou, J.; Kang, W.; Dong, Z.; Wang, H., Tocilizumab inhibits neuronal cell apoptosis and activates STAT3 in cerebral infarction rat model. Bosn J Basic Med Sci 2016, 16 (2), 145-50.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们



















