细胞实验
Cell culture and drug exposition[1]
Human RPE cells (ARPE-19 cells) werecultured in DMEM/F12 supplemented with 10% fetal bovine serum (FBS) and 1%penicillin/streptomycin (full standard medium), and passages 22 through 25 wereused for experiments. Cell authentication was successfully performed by shorttandem repeat (STR) DNA typing to proof the RPE cell identity.
Cells were induced to polarize by plating(2 x 104 cells) on laminin coated 0.4 lm-pore 12-well transwellfilters, and maintained in DMEM/F12 supplemented with only 1% FBS and 1%penicillin/streptomycin (reduced serum standard medium). Media were changedtwice a week for4 weeks before use in experiments.
After polarization, RPE cells in the upperchamber were incubated with defined clinical doses of ranibizumab (0.125 mg/ml)and unspecific Fab-fragment (isotype control, human IgG Fab fragment, 0.125mg/ml), which were labeled with Dylight650 (kex/kem = 652 nm/672 nm) accordingto the manufacturer’s protocol, or drug-free reduced serum standard medium(untreated control) for 1 h, before being replaced by fresh reduced serummedium. After either 24 h or 72 h the media of the upper and lower compartmentwere collected separately and stored at -80 oC. The same procedurewas conducted, but with the addition of a neutralizing anti-human VEGFR-2antibody (50 ng/mL) to block VEGFR-2 of the RPE cells prior to drug application.
Cellproliferation and oxidative stress
ARPE-19 cells were plated (2 x 104 cells) on laminin-coated glass cover slips in 12-well plates and left to adherein full standard medium for 24 h. Then the culture medium was replaced withfresh reduced serum standard medium for another 24 h. Thereafter, RPE cellswere incubated with 0.125 mg/ml ranibizumab, unspecific Fab-fragment (isotype control),or drug-free reduced serum standard medium (untreated control) for 6 h. Thenculture media were replaced by fresh culture media with or without 0.6 mM H2O2 for 18 h. To evaluate cell proliferation, the number of cell nuclei was countedby staining them with DAPI (1μg/ml) for 10 min at RT. Three fluorescence images were captured ofeach cover slip and analyzed by counting the number of DAPI-positive cellnuclei using ImageJ software.
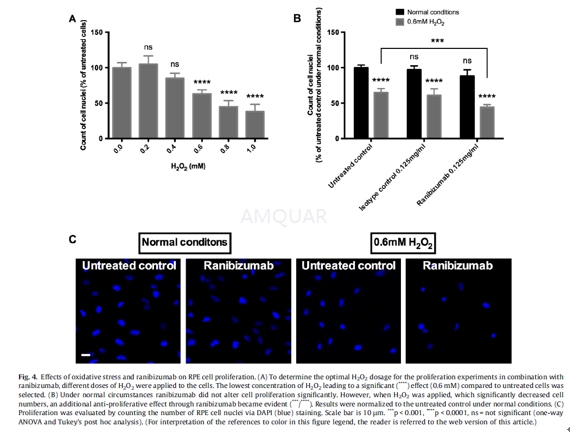
动物实验
Animals and study protocol[2]
Ten cynomolgus monkeys (Macacafascicularis, aged 3 to 8 years) were raised under standard conditions. Fulldetails are shown in Table 1.
The naive Cynomolgus fascicularis monkeys arefrom a closed breeding colony. Tests for TB, B-Virus, SIV, SRV, STLV, arecarried out during export and import quarantine. Additionally tests for TB,B-Virus, SIV, SRV, STLV are carried out regularly for all animals on siteregardless if they are in studies or not.
Cynomogus monkeys are housed in socialgroups before and during studies.
The animal live under a 12 hour dark lightcycle, have ad libidum access to water and food provided twice daily, lab dietplus fresh fruit. The foremost enrichment is social housing. Additionallymirrors, wooden trunks, balls are supplied as a standard. Further enrichmentdevices are available and made available on a rotating scheme.
The animal welfare officer on siteregularly checks the housing and handling conditions.
Since animals are housed in groups duringthe study the individuals are not randomised to the dose group, but rather astable group of animals created a long time before the study.
Both eyes of four animals wereintravitreally injected with ranibizumab and another four animals withaflibercept. The doses of anti-VEGF agents were the same as clinically used inhumans and as provided and recommended by the manufacturers: 2 mg ofaflibercept and 0.5 mg of ranibizumab, respectively.
All injections were carried out in themorning, following slight sedation with ketamin and diazepam. One and sevendays after intravitreal injection, the animals were sacrificed under generalanaesthesia and the kidneys were removed (except the left kidney of a monkeythat was sacrificed on day seven after injection of aflibercept showingaplasia). The left kidney of each animal was prepared for immunohistochemistry,specimens of the right kidneys served for electron microscopy as describedbelow. One untreated monkey sacrificed on day seven and one monkey injectedwith aflibercept’s vehicle and sacrificed one day after injection served ascontrols. Blood samples were taken before injection (predose) and on days oneand seven after injection. Platelet-poor plasma was prepared by centrifugation.
Immunohistochemistry
Sections (4μm) were cutfrom formalin-fixed, paraffin embedded tissue and mounted on superfrost Plusmicroscope slides.
The slides were deparaffinised andrehydrated, and heat induced epitope retrieval in TRIS EDTA (pH 9) using apressure cooker was performed. After two washing steps in TBS (0.5% TWEEN)immunohistochemical staining of VEGF was performed according to theinstructions provided by the manufacturer in a humid chamber. The slides wereincubated for 60 min with the primary mouse anti-VEGF antibody (1:50, DAKO) at37°C and then processed using the DAKO REAL Detection System AlkalinePhosphatase/RED kit rabbit/mouse, then counterstained with hematoxylin andcovered. The same procedure was performed for immune reactivity analysisagainst ranibizumab and aflibercept using respectively a primary mouse antibodyagainst the human IgG-Fabfragment (Dianova, 1:100) and a primary mouse antibodyagainst the human IgGFc- fragment (Dianova, 1/150). These samples were used forthe quantification and normalisation of VEGF or ranibizumab/afliberceptstainings.
Additionally an immune reactivity analysisusing fluorescent antibodies was performed. A mouse antibody against the humanIgG-Fab-fragment of IgG (dilution 1:400) and a goat anti-mouse alexa488 labelledsecondary antibody (dilution 1:400) were used for ranibizumab staining. Goatanti-human IgG-Fc antibody (dilution 1:200) and a donkey anti-goat alexa488labelled secondary antibody (dilution 1:500, molecular probes) were used foraflibercept staining.

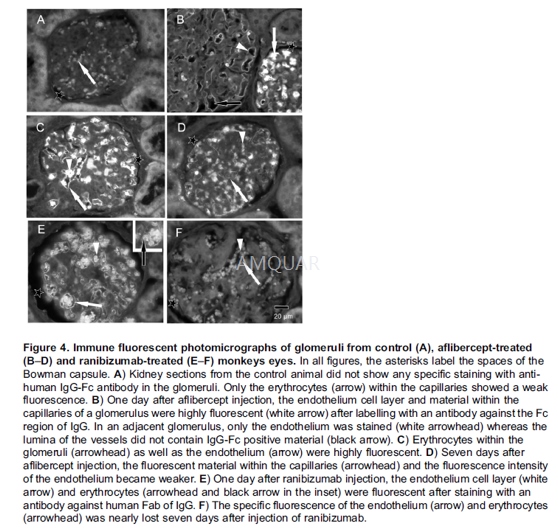
参考文献
[1] Ranjbar, M.; Brinkmann, M. P.; Tura, A.; Rudolf, M.; Miura, Y.; Grisanti, S., Ranibizumab interacts with the VEGF-A/VEGFR-2 signaling pathway in human RPE cells at different levels. Cytokine 2016, 83, 210-6.
[2] Tschulakow, A.; Christner, S.; Julien, S.; Ludinsky, M.; van der Giet, M.; Schraermeyer, U., Effects of a single intravitreal injection of aflibercept and ranibizumab on glomeruli of monkeys. PLoS One 2014, 9 (11), e113701.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度



















