-
生物活性
Pertuzumab,a monoclonal antibody against ERBB2 (HER2), is used in the treatment of HER2positive breast cancer.
-
体外研究
-
体内研究
-
激酶实验
Flow cytometric analysis[1]
Indirect surface staining was performed onSKB-R3 and MDAMB-231 cells. After trypsinization, cells were washed with PBScontaining 2% FBS and incubated with 100μL of 0.1μg/mLof purified Pertuzumab mAbs and Perjeta as primary antibodies at 4 oCfor 1 h. After incubation, Cells were recovered by centrifugation at 300xg for5 min, washed twice and Pertuzumab at the cell surface was detected using FITC conjugatedmouse anti-human IgG antibody and analyzed by flow cytometry using a FACSCalibur.
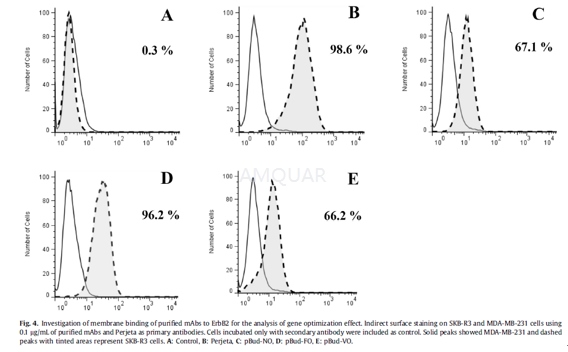
-
细胞实验
Cell lines and cell culture[2]
CHOFUT8−/− cells were generated by CRISPR/Cas9. The CHO cell lines containingantihuman HER2 antibody gene was cultured in DMEM/F12 selective mediumsupplemented with 10% fetal bovine serum, 1μg/mL Zeocin and20μg/mL Blasticidin, and incubated at 37oC, 5% CO2.Stably expressing cell pools were cultured in CD-CHO medium in 1 L shake flaskfor 5 days to collect supernatants. In ADCC and CDC assays, SK-BR-3 wascultured in DMEM medium supplemented with 10% FBS.
Antibody-dependentcellular cytotoxicity (ADCC) assays
ADCC activity was determined by the LDHrelease assay. SK-BR-3 was chosen as target cells (T). Human PBMCs purifiedfrom healthy donor using Lympholyte-H were used as effector cells (E). E/Tratio was determined by mix SK-BR-3 with PBMCs in the presence of antihumanHER2 antibodies. Further, PBMCs mixed with SK-BR-3 at an E/T ration of 50/1,and incubated with different pertuzumab concentrations. After 4 h incubation at37 °C., the plate was centrifuged at 250 g for 5 min and transferred 50μL ofthe supernatants to another new 96-well plate. According the manufacturer’sinstructions, 50μL/well substrate was added to each well and incubates for 30min;the reaction was stopped and read the absorbance of 490 nm. Besides, effectorcells self-release, target cells self-release, target cells maximum release, blankcontrol group and positive control group were also set.

-
动物实验
HBCx-19 mouse xenograft model[3]
All animal experiments were carried out inaccordance with ethical and local authority guidelines.
An ER+/HER2-low/HER3+ human breast cancertumor fragment derived from a metastasis of a lobular carcinoma (HBCx-19) wasobtained with informed consent from a patient treated at a cancer center.Tissue fragments were subcutaneously transplanted onto 5-10 female outbredathymic (nu/nu) donor mice (HSD:Athymic Nude-Foxn1nu). Donor mice weresacrificed when tumors reached 1000-2000 mm3 in volume and tumorsaseptically excised and dissected. Necrotic areas were removed and theremaining tumors were cut into fragments measuring approximately 20 mm3 andtransferred in culture media before grafting. Mice were anaesthetized with 100mg/kg ketamine hydrochloride and 10 mg/kg xylazine, the skin was sterilizedwith chlorhexidine solution, and incised at the level of the interscapularregion. A 20 mm3 tumor fragment was placed in the subcutaneous tissue and theskin was closed with clips. All mice from the same experiment were implanted onthe same day.
Animals were delivered to the laboratory 7daysbefore the experiments during which time they were acclimatized to laboratoryconditions. Mice were housed inside individually ventilated cages (IVC) under alight-dark cycle (14-hour circadian cycle of artificial light) and controlledroom temperature and humidity. Food and water were provided ad libitum. Animalswere controlled daily for clinical symptoms and detection of adverse effects.Mice received estrogen diluted in drinking water (β-estradiol, 8.5mg/L), from the date of tumor implant to the end of the study.
For investigation of lumretuzumab andpertuzumab, tumor-bearing animals were treated with vehicle (20 mM histidinemonohydrochloride monohydrate, 140 mM NaCl, pH 6) or lumretuzumab (10 mg/kgintraperitoneally [i.p.]) or pertuzumab (15 mg/kg i.p. with a twofold loadingdose) as single agents or in combinations. For investigation of the additionalbenefit of adding fulvestrant to lumretuzumab and pertuzumab, the same animalmodel was used and was treated with vehicle, single-agent lumretuzumab (3 mg/kgi.p.), single-agent pertuzumab (3 mg/kg i.p.), single-agent fulvestrant (50mg/kg intramuscularly [i.m.]) or with the reported combinations. Treatmentswere given weekly for six weeks or for up to 9 weeks startingat randomizationwhen median tumor size was approximately 100-150 mm3. Tumor volumewas measured using calipers every 3±4 days and the percentage tumor growthinhibition relative to control animals was calculated.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Ramezani, A.; Mahmoudi Maymand, E.; Yazdanpanah-Samani, M.; Hosseini, A.; Toghraie, F. S.; Ghaderi, A., Improving Pertuzumab production by gene optimization and proper signal peptide selection. Protein Expr Purif 2017, 135, 24-32.
[2] Luo, C.; Chen, S.; Xu, N.; Wang, C.; Sai, W. B.; Zhao, W.; Li, Y. C.; Hu, X. J.; Tian, H.; Gao, X. D.; Yao, W. B., Glycoengineering of pertuzumab and its impact on the pharmacokinetic/pharmacodynamic properties. Sci Rep 2017, 7, 46347.
[3] Collins, D.; Jacob, W.; Cejalvo, J. M.; Ceppi, M.; James, I.; Hasmann, M.; Crown, J.; Cervantes, A.; Weisser, M.; Bossenmaier, B., Direct estrogen receptor (ER) / HER family crosstalk mediating sensitivity to lumretuzumab and pertuzumab in ER+ breast cancer. PLoS One 2017, 12 (5), e0177331.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们



















