-
生物活性
Panitumumab,a fully human and less immunogenic anti-EGFR monoclonal antibody, is widelyused in patients with metastatic colorectal cancer.
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
Cell Lines and Reagents[1]
CRC lines, HCT-116, T84, LoVo (all KRAS G13Dmutant), SW480 (KRAS G12V mutant), LIM1215 CRC line (KRAS WT) were purchased.The cell lines were cultured in 75-mL tissue culture flasks in RPMI 1640 mediumsupplemented with 10% fetal bovine serum, 100 U/mL penicillin, 100 μg/mLstreptomycin, and 200 μg/mL glutamine at 37°C and 5% CO2. The cell lines weretested with the MycoAlert mycoplasma detection kit and were found to be free ofmycoplasma contamination throughout the experimental procedure.
MonoclonalAntibodies
Cetuximab at 5 mg/mL and panitumumab at 20mg/mL were prepared.
ProliferationAssay and Optimization of Antibody Concentrations
The CellTiter 96® AQueous nonradioactive cellproliferation assay kit was used to assess the resistance and sensitivity ofthese CRC lines to the monoclonal antibodies. The cells were seeded into96-well plates at 5 x 103 cells/mL in a total volume of 100 μLmedium and incubated for 24 hours in a 5% CO2 humidified atmosphere at37°C. The cells were treated in triplicate with cetuximab or panitumumab atconcentrations of 0.5–10μg per well, to determine the optimal concentrations for theproliferation assay. Another set of cells on the plate was treated intriplicate with 10μg of monoclonal mouse IgG1 or IgG2A isotype control antibody as anegative control. The plates were incubated for 72 hours at 37 °C in a 5% CO2 incubator, and all wells were then treated with 20μL of MTS/ PMSsolution and incubated for a further1.5 hours. The absorbance was read with theFluostar Optima instrument at a wavelength of 490 nm. Proliferation wasrecorded as a percentage of that obtained for the isotype controlantibody-treated cells (100%).
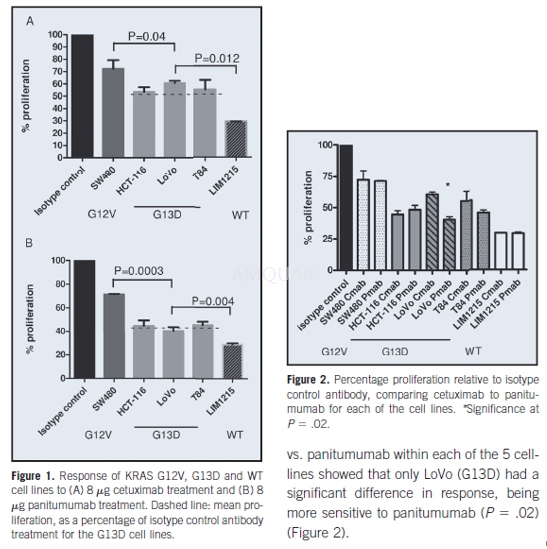
-
动物实验
Subcutaneous tumor xenograft models[2]
Female BALB/ cA Jcl-nu/nu (nude) mice andC.B17/Icr-scid/scid Jcl (SCID) mice were maintained under specificpathogen-free conditions. LIM1215 cells (5 x 106) mixed withMatrigel were inoculated subcutaneously into the right flank of six- toseven-week-old SCID mice. Once established, the tumors were surgically excised,and smaller tumor fragments (about 2 mm in diameter) were subcutaneouslyimplanted in the rightflank of SCID mice. To establish the patient-derived colontumor xenograft (PDX) model, COL-01-JCK PDX line was obtained and tumor fragmentswere implanted into the right flank of female nude mice. The mice wererandomized when the mean tumor volume reached approximately 50–200 mm3.The mice were then treated with the vehicle (0.5% hydroxypropyl methylcellulosesolution or saline), panitumumab (intraperitoneally), TAS-102 [a mixture of FTDand TPI at a molar ratio of 1:0.5 (orally)], or panitumumab/ TAS-102combination for 2 weeks. The tumor volumes were measured twice weekly withVernier calipers and calculated as the length x width2 x 0.5. The treated/controlratio (T/C, %) was calculated by dividing the change in tumor volume in thedrug-treated mice by that in the vehicle-treated control mice. The percentage oftumor regression was calculated as follows: tumor regression (%) at day X = [1-(tumorvolume at day X/ tumor volume at day 0)] x 100. Statistical comparisons oftumor volumes and body weights were made using Dunnett’s multiple comparisontests; P < 0.05 was considered statistically significant.

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Kumar SS, P. T., Mohyieldin O, Borg M, Townsend A, Hardingham JE, KRAS G13D Mutation and Sensitivity to Cetuximab or Panitumumab in a Colorectal Cancer Cell Line Model. Gastrointest Cancer Res 2014, 7 (1), 23-26.
[2] Baba, Y.; Tamura, T.; Satoh, Y.; Gotou, M.; Sawada, H.; Ebara, S.; Shibuya, K.; Soeda, J.; Nakamura, K., Panitumumab interaction with TAS-102 leads to combinational anticancer effects via blocking of EGFR-mediated tumor response to trifluridine. Mol Oncol 2017.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们


















