-
生物活性
Omalizumab (rhuMAb-E25),an anti-IgE antibody, inhibits the interaction between IgE and FcϵRI, is thefirst targeted biologic therapeutic approved for the treatment of moderate-to-severepersistent allergic asthma (AA).
-
体外研究
Omalizumab complexes circulating IgE, blocking IgE bindingto high affinity epsilon Fc receptors (FcεR1) on mast cells and basophils.[1] TheIgE:omalizumab complexes trapped increasing amounts of antigen with increasing(a) concentration of IgE, (b) proportion of antigen-specific IgE in total IgE,and (c) concentration of total immune complexes.[2]
-
体内研究
-
激酶实验
Analysis of the binding of IgE:omalizumab IC andantigen with Protein A column and SDS-PAGE and Western blotting analysis.[2]
To detect the interaction of IgE:omalizumabIC with antigen, Protein A-Sepharose 4B beads were used to absorb omalizumaband its associated proteins. Subsequently, the absorbed proteins were subjectedto (i) SDS-PAGE and (ii) Western blotting analysis. For these analyses, SE44-IgE and omalizumab were mixed at a molar ratio of1:2 and incubated at roomtemperature for 30 min. Next, 500 μl of (R15K)8-ova at 2 mg/ml wasadded and incubated at 37 °C for 30 min. The mixture was passed through aProtein A-Sepharose 4B column and the absorbed protein was processed for twoseparate 10% SDS-PAGE gels. In one SDS gel, the resolved proteins were stainedwith Coomassie blue. The proteins in the other SDS gel were transblotted onto aPVDF membrane (Hybond-P), and the presence of (R15K)8-ova wasidentified by biotinylated SE44 IgE and avidin conjugated with horseradishperoxidase.
-
细胞实验
Histamine Releasefrom Peripheral Blood Basophils[3]
Human venous whole blood from healthydonors was collected and anticoagulated in heparin. Blood specimens werediluted in Hanks’ basic salt solution (HBSS) containing 1% BSA andpresensitized with human plasma containing ragweed-specific IgE (I64ng/ml). Theeffect of rhumAb-E25 on ragweed-specific-IgE-sensitized basophils wasdetermined by addition of cither 100 ng/ml ragweed allergen or 10μg/mlanti-IgE mAbs: rhumAb-E25 or MAE1, (an anti-IgF. mAb which recognizesreceptor-bound IgE). The cells were incubated for 30 min. at 37°C in ahumidified 5% CO2 incubator, placed on ice to terminate histaminerelease, and supernatants were assayed for histamine content by immunoassay.
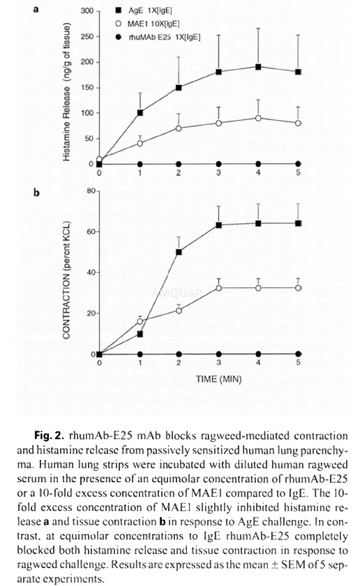
-
动物实验
Cutaneous Sensitivity to Ragweed[3]
Cynomolgus monkeys were sensitized biweeklyfor 31 weeks by intramuscular injection with ragweed extract (40μg/kg)mixed 1:1 with alum (20 mg/ml). Skin test reactivity was measured by injecting 1ml/kg of 0.5% Evans Blue dye followed by intradermal injections of serial10-fold dilutions of ragweed extract (0.001-1μg/site) orexcipient control. Each injection site was observed for redness, swelling, andblue color, and scored (0=no response, 1=modcrate, 2=severe reaction).Quantitative skin tests were begun on these animals at week 18 to determinebaseline skin test reactivity prior to rhumAb-E25 administration. This is takenas the time zero point for data presentation. rhumAb-E25 (10-50 mg/kg) wasadministered only on weeks 2 and 6 relative to this first quantitative skintest. Antigen boosts continued biweekly during the treatment period. Changes inconcentration of ragweed required to produce a 2 reaction were scored at thetimes indicated in the results. Antigen-specific IgE titers were also measuredby ELISA.
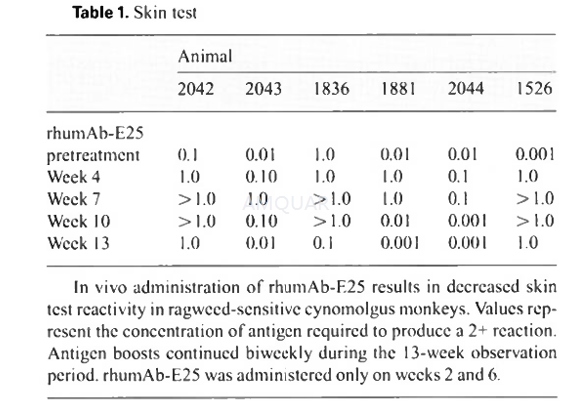
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Hamilton, R. G.; Marcotte, G. V.; Saini, S. S., Immunological methods for quantifying free and total serum IgE levels in allergy patients receiving Omalizumab (Xolair) therapy. Journal of Immunological Methods 2005, 303 (1-2), 81-91.
[2] Hsu, C.-L.; Shiung, Y.-Y.; Lin, B.-L.; Chang, H.-Y.; Chang, T. W., Accumulated immune complexes of IgE and omalizumab trap allergens in an in vitro model. International Immunopharmacology 2010, 10 (4), 533-539.
[3] Shields RL, W. W., Zioncheck K, O'Connell L, Fendly B, Presta LG, Thomas D, Saban R, Jardieu P, Inhibition of allergic reactions with antibodies to IgE. Int Arch Allergy Immunol 1995, 107 (1-3), 308-12.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们


















