-
生物活性
Ofatumumab,a humanized anti-CD20 monoclonal antibody, has been proven to be effective inthe treatment of relapsed, refractory CLL.
Ofatumumabinduceds complement-dependent cytotoxicity (CDC) in SU-DHL4 and Z138 cell lineswith EC50 values of 0.4 nmol/L and 0.7nmol/L, respectively. [1]
-
体外研究
-
体内研究
-
激酶实验
Evaluation of C1q binding[1]
The binding of the human complementcomponent C1q to each mAb was assessed by ELISA. Serial dilutions of theantibodies were immobilized on a MaxiSorp 96-well plate. Free binding siteswere blocked with PBS containing 3% bovine serum albumin followed by incubationwith C1q (2.2μg/mL) at room temperature for 90 minutes. Plates were washed andbound C1q were detected using polyclonal rabbit anti-human C1q with horseradishperoxidase–conjugated polyclonal goat anti-rabbit Fc and 2, 20- azino-bis(3-ethylbenzothiazoline-6-sulfonicacid (ABTS). Measurements were carried out using an automated microplate reader(405 nm/490 nm).

-
细胞实验
Culture settings[2]
All cultures were maintained in RPMI 1640medium containing 10% (v/v) heat inactivated foetal calf serum (FCS) andantibiotics (streptomycin 50 mg/ml, penicillin50 IU/ml), at 37oC, 5%CO2, fully humidified.
Assessmentof drug cytotoxicity
Cytotoxicity (cell viability) was assessedbased on propidium iodide (PI) staining. After incubation with drugs, cellswere washed twice in phosphate buffered solution (PBS) and then re-suspended in0.5 ml of 10 μg/ml PBS/PI solution. After 10 min of staining (room temperature, inthe dark) cell fluorescence was measured by flow cytometry, using the FL3 (red)standard fluorescent filter. The viable cells were defined as PI-negative.
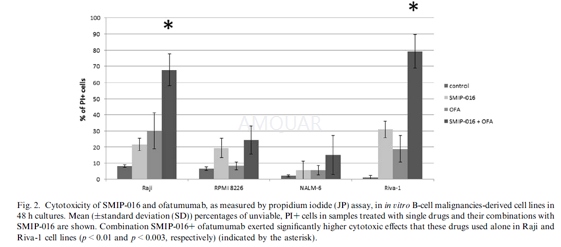
Ofatumumab and rituximab in vitro testing[3]
Vitally frozen cells were uniformly usedfor the testing. For metabolic WST-1 assay measuring cellular viability, cellswere seeded in 96-well plates in quadruplicates (500,000 cells per well) andcultivated 48 h in the presence of 10, 20, and 30μg/mL of ofatumumab(OFA),rituximab (RTX) and nonspecific immunoglobulin (IgG) as a negative control.Twenty percent active human serum was added to allow CDC and the longercultivation time was chosen to employ potential other cell death mechanisms.Final cell viability was assessed at 450 nm on SLT. Spectra reader. The testingnot involving active human serum was performed in the same manner.
Propidium iodide (PI) was used to evaluatecell death using flow cytometry. Cells were treated with10μg/mLMabs in the same experimental setting as for WST-1 assay. PI binds to doublestranded DNA, but is excluded from viable cells with intact plasma membranes.PBMNC, 300,000 per tube, were stained according to manufacturer’s instructions.Data acquisition with subsequent analysis was performed on BD FACSAria III usingthe BD FACSDiva software.


-
动物实验
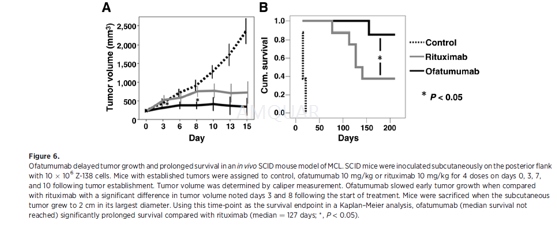
In vivo effects ofofatumumab and rituximab in mantle cell lymphoma[4]
For in vivo experiments, 6- to 8-week-old SCID mice werehoused and maintained in laminar flow cabinets or micro isolator units andprovided with sterilized food and water. Six- to 8-week-old SCID mice wereinoculated subcutaneously on the posterior flank with10x106 Z-138cells suspended in Matrigel. Mice were monitored for establishment of asubcutaneous tumor nodule. Tumor volume was determined by caliper measurement.Once the tumor volume reached approximately 250 mm3, mice wereassigned to either a control group, ofatumumab (10 mg/kg), or rituximab (10mg/kg). Monoclonal antibodies were delivered IV via tail vein injection ondays+0, +3,+7, and +10 following tumor engraftment. Tumor volume was monitoredby serial caliper measurement. Mice with a tumor exceeding 2 cm in largestdiameter were sacrificed as per RPCI IACUC requirements. Tumor size >2 cm inlargest diameter was used as the survival endpoint in a Kaplan–Meier analysis.Differences in outcomes between treatment groups were compared by log-rankanalysis.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Herter, S.; Herting, F.; Mundigl, O.; Waldhauer, I.; Weinzierl, T.; Fauti, T.; Muth, G.; Ziegler-Landesberger, D.; Van Puijenbroek, E.; Lang, S.; Duong, M. N.; Reslan, L.; Gerdes, C. A.; Friess, T.; Baer, U.; Burtscher, H.; Weidner, M.; Dumontet, C.; Umana, P.; Niederfellner, G.; Bacac, M.; Klein, C., Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013, 12 (10), 2031-42.
[2] Smolewski, P.; Robak, P.; Cebula-Obrzut, B.; Misiewicz, M.; Medra, A.; Majchrzak, A.; Witkowska, M.; Stromatt, S.; Robak, T., Pro-apoptotic effect of an anti-CD37 scFv-Fc fusion protein, in combination with the anti-CD20 antibody, ofatumumab, on tumour cells from B-cell malignancies. Eur J Cancer 2014, 50 (15), 2677-84.
[3] Sebejova, L.; Borsky, M.; Jaskova, Z.; Potesil, D.; Navrkalova, V.; Malcikova, J.; Sramek, M.; Doubek, M.; Loja, T.; Pospisilova, S.; Mayer, J.; Trbusek, M., Distinct in vitro sensitivity of p53-mutated and ATM-mutated chronic lymphocytic leukemia cells to ofatumumab and rituximab. Exp Hematol 2014, 42 (10), 867-74 e1.
[4] Barth, M. J.; Mavis, C.; Czuczman, M. S.; Hernandez-Ilizaliturri, F. J., Ofatumumab Exhibits Enhanced In Vitro and In Vivo Activity Compared to Rituximab in Preclinical Models of Mantle Cell Lymphoma. Clin Cancer Res 2015, 21 (19), 4391-7.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们





















