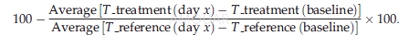-
生物活性
Obinutuzumab (Gazyva) is a unique,third-generation, fully humanized glycoengineered IgG1 type II anti-CD20monoclonal antibody monoclonal antibody, approved in chronic lymphocyticleukemia (B-CLL) and follicular lymphoma (FL).
Obinutuzumab (Gazyva) induceds complement-dependent cytotoxicity (CDC) in SU-DHL4 and Z138cell lines EC50 values of 6.3 nmol/L and >200 nmol/L, respectively.[1]
-
体外研究
-
体内研究
-
激酶实验
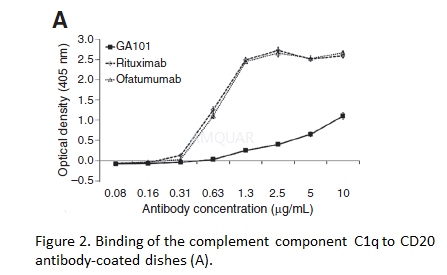
Evaluation of C1q binding[1]
The binding of the human complementcomponent C1q to each mAb was assessed by ELISA. Serial dilutions of theantibodies were immobilized on a MaxiSorp 96-well plate. Free binding siteswere blocked with PBS containing 3% bovine serum albumin followed by incubationwith C1q (2.2μg/mL) at room temperature for 90 minutes. Plates were washed andbound C1q were detected using polyclonal rabbit anti-human C1q with horseradishperoxidase–conjugated polyclonal goat anti-rabbit Fc and 2, 20- azino-bis(3-ethylbenzothiazoline-6-sulfonicacid (ABTS). Measurements were carried out using an automated microplate reader(405 nm/490 nm).
-
细胞实验
Cell lines[2]
Human B-lymphoma Raji (CD20+)and Loucy T-cell acute lymphocytic leukaemia (T-ALL) (CD20- negativecontrol) cell lines; precursor-CD20+ lymphoblastic leukaemia U698-M(CD20+) and pre-B-ALL, Nalm-6 cell lines rituximab-resistant celllines Raji2R and Raji4RH; K562-mbIL15-41BBL cells were maintained in RPMI1640media supplemented with 10% fetal calf serum (FCS) and 2 mmol/l glutamine at37 °C, 5% CO2.
Celldeath assays
The optimum effective dose and timeresponse of obinutuzumab was first determined by treating CD20+ andCD20- tumour targets with a dose escalation of obinutuzumab (1–100μg/ml,for 24, 48 and 72 h). Raji, Raji2R, Raji4RH, Loucy and U698-M cells were seededin 96-well plates at106/ml, with obinutuzumab (1–00μg/ml),rituximab (100μg/ml) or human polyclonal IgG (isotype control) and incubated at37°C with 5% CO2 for 24–72 h. Cells were washed, re-suspended in 10%RPMI medium and stained with annexin V-PE/7-Aminoactinomycin D (7-AAD) todetect loss of plasma membrane integrity and with CD19-FITC. Cell death wasdetermined by flow cytometry. Cells were evaluated within the lymphocyte gate;approximately 20000 events were captured and analysed for each sample. Theoptimum dose and schedule for obinutuzumab was determined to be 100μg/mlfor 48 h and was used for all future in vitro studies.
Cellproliferation assay
Tumour cell lines were treated with 100μg/mlobinutuzumab, rituximab or isotype control as described above. Alamar blue dyewas added at 10% sample volume, and incubated for 1–4 h at 37°C. Cell viabilitywas determined by fluorescence spectrophotometer at 580 nm, excitation emission600 nm. Cell proliferation was calculated as the percentage of fluorescence ofcells with respect to untreated control cells, after subtracting for backgroundfluorescence in the absence of cells.

-
动物实验
In vivo antitumor activity[1]
The human DLBCL cell line SU-DHL4 wassubcutaneously inoculated (5 x106 cells) with Matrigel into theright flank of 4- to 5-week-old female severe combined immunodeficient (SCID)beige mice maintained under the standard conditions. Female SCID beige mice, 4to 5 weeks of age, were maintained under specific pathogen-free conditionsaccording to guidelines. Continuous health monitoring was carried out on aregular basis, with daily monitoring of clinical symptoms and adverse effects.Primary tumor volume (TV) was calculated as follows [TV = (length x width2)=2], where "length" and "width" are the long and shortdiameters of the tumor mass in millimeters. Antitumor activity was assessed by calculatingtumor-growth inhibition (TGI) based on medians by using the following formula:
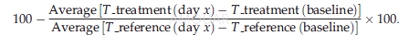
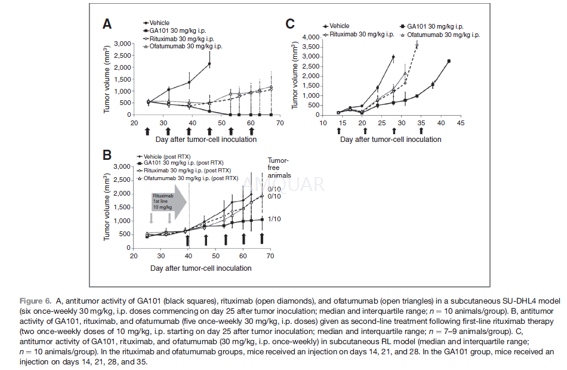
At 25 days after cell transplantation, 10animals with established subcutaneous SU-DHL4 tumors (>500 mm3) wererandomized to vehicle control, single-agent GA101, rituximab, or ofatumumab[all 30 mg/kg intraperitoneally (i.p.)]. Treatment commenced 25 days aftertumor-cell inoculation (median tumor volume, 504–571 mm3), with administrationrepeated on days 32, 39, 46, 53, and 60. TGI was assessed on day 46 aftertumor-cell inoculation, and animals were observed until day 67 to evaluatetumor status. To evaluate second-line antitumor activity, mice bearing tumorswith a median volume of 504 to 571 mm3 received rituximab (10 mg/kg,i.p.) on days 25 and 32 after tumor-cell inoculation (median tumor volume,626–633 mm3) and were randomized to vehicle control, singleagent GA101,rituximab, or ofatumumab (all 30 mg/kg, i.p.). Second-line treatment wasadministered on study days 39, 46, 53, 60, and 67, with second-line antitumor activityevaluated on day 63 after tumor-cell inoculation. The human indolent NHL cellline RL was subcutaneously inoculated (10 x 106 cells) into theright flank of the mice, and after 14 days, 10 animals with established subcutaneousRL tumors (median volume, 150mm3) were randomized to each group:vehicle control, single-agent GA101, rituximab, and ofatumumab (all 30 mg/kg,i.p. once-weekly over 4 weeks). TGI was assessed on day 28 after tumor-cellinoculation. Raw data from the RL experiment were processed in the statisticssoftware SAS-JMP version 8.0.2.2 using the menu RocheTools3.1. Primary tumorvolume and antitumor activity were calculated by using the established methods.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Herter, S.; Herting, F.; Mundigl, O.; Waldhauer, I.; Weinzierl, T.; Fauti, T.; Muth, G.; Ziegler-Landesberger, D.; Van Puijenbroek, E.; Lang, S.; Duong, M. N.; Reslan, L.; Gerdes, C. A.; Friess, T.; Baer, U.; Burtscher, H.; Weidner, M.; Dumontet, C.; Umana, P.; Niederfellner, G.; Bacac, M.; Klein, C., Preclinical activity of the type II CD20 antibody GA101 (obinutuzumab) compared with rituximab and ofatumumab in vitro and in xenograft models. Mol Cancer Ther 2013, 12 (10), 2031-42.
[2] Awasthi, A.; Ayello, J.; Van de Ven, C.; Elmacken, M.; Sabulski, A.; Barth, M. J.; Czuczman, M. S.; Islam, H.; Klein, C.; Cairo, M. S., Obinutuzumab (GA101) compared to rituximab significantly enhances cell death and antibody-dependent cytotoxicity and improves overall survival against CD20(+) rituximab-sensitive/-resistant Burkitt lymphoma (BL) and precursor B-acute lymphoblastic leukaemia (pre-B-ALL): potential targeted therapy in patients with poor risk CD20(+) BL and pre-B-ALL. Br J Haem
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们