-
生物活性
PD-1binding abilities of nivolumab[1]
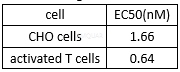
Theinteraction inhibition of nivolumab between PD-1 and its ligands[1]
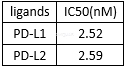
-
体外研究
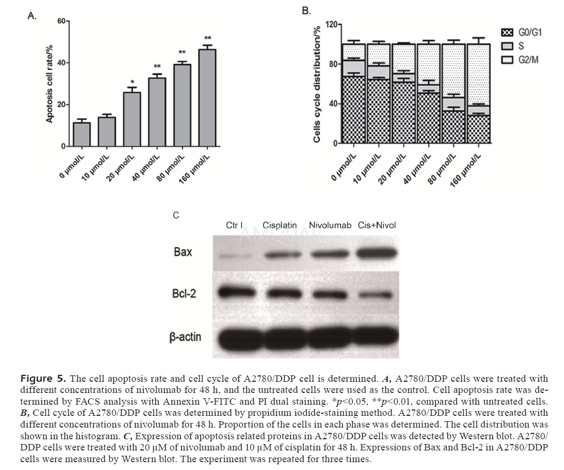
Nivolumabbinds specifically to PD-1 and not to other immunoglobulin superfamilyproteins, such as CD28, CTLA-4, ICOS, and BTLA. Nivolumab can, at very lowconcentrations (~1.5ng/mL), enhance T-cell reactivity in the presence of aT-cell receptor stimulus. However, nivolumab does not cause nonspecificlymphocyte activation.[1] Nivolumabincreases the anti-tumor effects of cisplatin in A2780/DDP cells. Besides, thecombined treatment effectively reversed cisplatin resistance in PROC cells.Also, nivolumab induced cell apoptosis and cell-cycle arrest in G0/G1 phase inPROC cells.[2]
-
体内研究
-
激酶实验
FACS analysis of nivolumab binding to PD-1mutants[3]
To obtain cell surface expressing PD-1fused with EGFP protein, the full-length PD-1 was cloned into the pEGFP-N1vector. The plasmids expressing the PD-1 mutants N49A, N58A, N74A or N116A weredone by site-directed mutagenesis. The plasmids were transfected into 293 Tcells, respectively. Cells were collected 48h after transfection andresuspended in PBS at 1x107cells /ml.
Then the 293 T cells expressing wild-typePD-1 or mutants were stained with nivolumabat room temperature for 30 min,washed three times with PBS and further stained with secondary APC-anti-humanIgG (Clone G18-145) for another 30 min. Cells were analysed by flow cytometrywith a BD FACS Aria II machine after washing.
-
细胞实验
Cell Lines[2]
The PROC cell lines A2780/DDP and SKOV3/DDPwere cultured in Dulbecco’s Modified Eagle Medium (DMEM) supplemented with 10%fetal bovine serum (FBS) at 37°C and 5% CO2
MTTAssay
The inhibitory effects of nivolumab forPROC cell lines A2780/DDP and SKOV3/ DDP cells were measured by MTT assay.Briefly, cells were cultured in cisplatin-free medium for3 days in advance. Thecells (1.0 × 104 cells/ well) were plated into 96-well plates. Cellswere allowed to attach to the bottom overnight, and then treated with differentconcentrations of nivolumab for 24 h, 48 h and 72 h, respectively. Controlcells received an equal amount of dimethyl sulfoxide (DMSO) only. Before test, 20μLof MTT (5 mg/mL) was added to each well and incubated for 4 h at 37°C in thedark. After removing the supernatant, formazan crystals formed were dissolvedin 100μL DMSO and mixed thoroughly before reading on a microplate reader. Theabsorbance was measured at 490 nm. All in vitro experiments were carried outthree times and calculated the averages.
Apoptosis
FACS was performed to detect the apoptosis rate.A2780/DDP cells (2×104 cells/well) were plated into 6-well plates.They were cultured for 6 to 8 h and treated with nivolumabfor 48 h. The cellswere analyzed after being treated with RNase and stained with Annexin v andpropidium iodide (PI) before test.
WesternBlotting
Western blots were performed. Briefly,total protein extract for each tissue sample or cell line was dissolved inlyses buffer and equal amounts of protein (60μg) were analyzed byimmunoblotting. Rabbit polyclonal antibodies against Bcl-2 and Bax and thehorseradish peroxidase- conjugated secondary antibody (goat-anti- rabbit) was used.

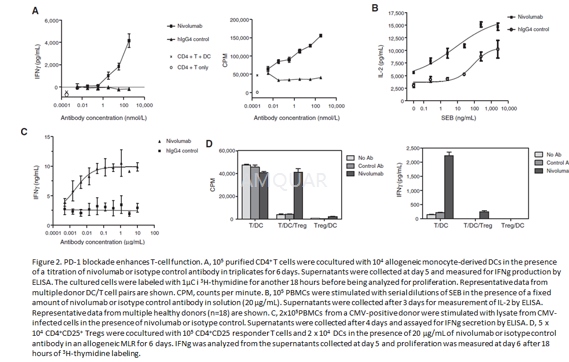
In vitro functional assays[1]
Mixedlymphocyte reaction
Dendritic cells (DC) were generated byculturing monocytes isolated from PBMCs using a monocyte purification kit invitro for 7 days with 500U/mL interleukin-4 (IL-4) and 250 U/mL GM-CSF. CD4þ Tcells (1x105) and allogeneic DCs (1x104) were coculturedwith or without dose titrations of nivolumab added at the initiation of theassay. After 5 days, IFNg secretion in culture supernatants was analyzed byELISA, and cells were labeled with 3H-thymidine for an additional 18 hours tomeasure T-cell proliferation.
Staphylococcalenterotoxin B stimulation of PBMCs
PBMCs from healthy human donors (N=18) werecultured for3 days with nivolumab or an isotype control antibody (20μg/mL) at the initiation of the assay together with serial dilutions ofstaphylococcal enterotoxin B. IL-2 levels in culture supernatants were measuredby ELISA analysis.
Antigen-specificrecall response in vitro
In a cytomegalovirus (CMV)-restimulationassay, 2 x 105 PBMCs from a CMV-positive donor were stimulated using lysate of CMV-infectedcells, with serial dilutions of nivolumab added at the initiation of the assay.After 4 days, supernatants were assayed for IFNg.
Suppressionassay with regulatory T cells
CD4+CD25+ regulatoryT cells (Tregs) and CD4+CD25- responder T cells werepurified from PBMCs (CD4+CD25+ Treg isolation kit). In anallogeneic mixed lymphocyte reaction (MLR) assay, Tregs (5 x 104)were cocultured with 1 x 105 responder T cells and 2 x 104 monocyte-derived DCs, with 20μg/mL nivolumab. After 5 days, IFNg production was assessed insupernatants, and cells were labeled with 3H-thymidine for an additional 18hours for proliferation analysis.
Antibody-dependentcell-mediated cytotoxicity
Antibody-dependent cell-mediatedcytotoxicity (ADCC) was assayed using the DELFIA Cell Cytotoxicity Kit. PBMCswere incubated overnight with 50 U/mL IL-2 and used as effector cells.Activated CD4+ T cells labeled with BATDA reagent were used astarget cells at an effector-to-target cell ratio of 50:1. Serial dilutions ofnivolumab or positive control [anti–major histocompatibility complex (MHC)class I antibody] were added; the cells were incubated for 3 hours at 37oC.To measure cytotoxicity, supernatant was mixed with Europium solution and readusing a RUBYstar Model 460 microplate reader.
-
动物实验
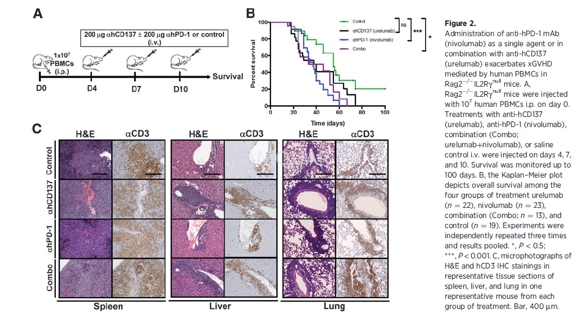
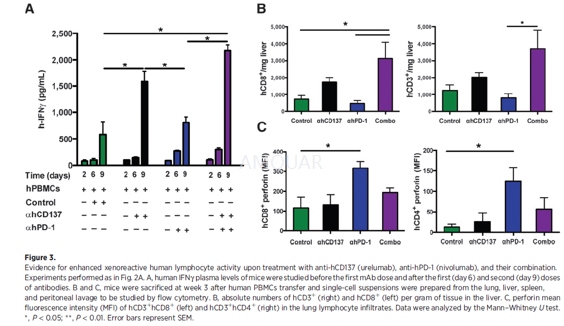
xGVHD model[4]
Three- to 4-week-old Rag2-/-IL2Rγnull mice were injected with1 x 107 human fresh PBMCs i.p. on day 0 of the experiment. Treatment with anti-hCD137(urelumab, 200 mg per injection), anti-hPD-1 (nivolumab, 200 mg per injection),Combo (nivolumab+urelumab, 200 mg per injection each), or saline control was administeredby intravenous (i.v.) injection on days 4, 7, and 10. Plasma samples wereprepared from blood collected in heparin tubes 1 day before the first injectionof treatment and after the first and second injection of the mAbs. Plasmasamples were stored at-80oC until use. Survival andxenograft-versus-host reaction was monitored daily up to 3 months. Animals thatdeveloped clinical symptoms of xGVHD (>15% weight loss, hunched posture, reducedmobility, fur loss, tachypnea) were sacrificed and an endpoint of survival wasrecorded. In some experiments mice were sacrificed on day 22 and lung and liverwere surgically removed, weighed, and processed for immunohistochemistry (IHC)and flow cytometry analysis.
Tumorexperiments
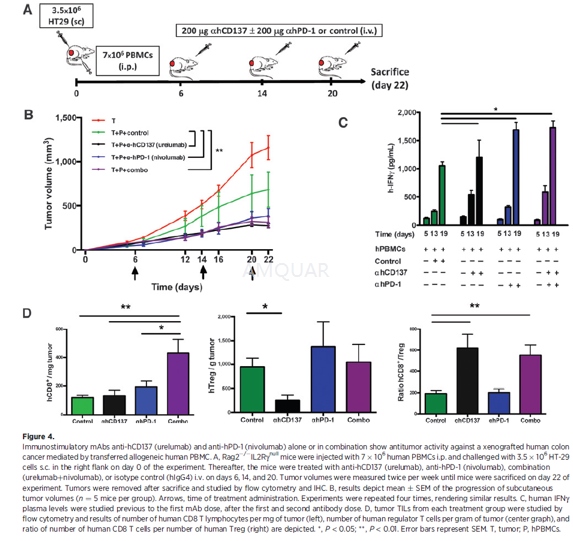
Three- to 4-week Rag2-/-IL2Rγnull mice old wereinjected with7x106 human fresh PBMCs i.p. and a total of 3.5 x106 HT29 human colon carcinoma cells were injected subcutaneously (s.c.) into theflank of Rag2-/-IL2Rγnull mice in 50 mL of PBS on day 0 of theexperiment. Mice were treated intravenously with antihCD137 (urelumab, 200 mgper injection), anti-hPD-1 (nivolumab, 200 mg per injection), Combo (nivolumab +urelumab, 200 mg per injection each), or isotype-matched control (200 mg of irrelevanthIgG4) on days 6, 14, and 20. Plasma samples were prepared from blood collectedin heparin 1 day before the first injection of treatment and after the firstand second injection. Plasma samples were stored at 80oC until use.Tumor growth was recorded every 3 to 4 days. Mice were sacrificed on day 22 ands.c. tumors were removed, weighed, and processed for IHC and flow cytometryanalysis.
In addition, 3- to 4-week-old Rag2-/-IL2Rγnull mice were injected i.p. with 2 or7 x 106 human PBMCs from a gastric cancer patient on day 0 ofexperiment. Explants (7 mm x7 mm) from the tumor of the same patient wereimplanted s.c. into the flank of Rag-/-IL2Rγnull mice on day 3 of the experiment. Mice were treated i.v. asindicated above on days 5, 12, and 19. Tumor growth was monitored every 3 to 4days. On day 22 of experiment mice were sacrificed, tumors removed, and studiedby quantitative immunofluorescence (QIF). Tumor growth analyses were limited to3 to 4 weeks because following this period of time mice started to show signsof xGVHD.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Wang, C.; Thudium, K. B.; Han, M.; Wang, X. T.; Huang, H.; Feingersh, D.; Garcia, C.; Wu, Y.; Kuhne, M.; Srinivasan, M.; Singh, S.; Wong, S.; Garner, N.; Leblanc, H.; Bunch, R. T.; Blanset, D.; Selby, M. J.; Korman, A. J., In vitro characterization of the anti-PD-1 antibody nivolumab, BMS-936558, and in vivo toxicology in non-human primates. Cancer Immunol Res 2014, 2 (9), 846-56.
[2] Sun LM, L. Y., Li W, Liu S, Liu HX, Li LW, Ma R, Nivolumab effectively inhibit platinum-resistant ovarian cancer cells via induction of cell apoptosis and inhibition of ADAM17 expression. Eur Rev Med Pharmacol Sci. 2017, 21 (6), 1198-1205.
[3] Tan, S.; Zhang, H.; Chai, Y.; Song, H.; Tong, Z.; Wang, Q.; Qi, J.; Wong, G.; Zhu, X.; Liu, W. J.; Gao, S.; Wang, Z.; Shi, Y.; Yang, F.; Gao, G. F.; Yan, J., An unexpected N-terminal loop in PD-1 dominates binding by nivolumab. Nat Commun 2017, 8, 14369.
[4] Sanmamed, M. F.; Rodriguez, I.; Schalper, K. A.; Onate, C.; Azpilikueta, A.; Rodriguez-Ruiz, M. E.; Morales-Kastresana, A.; Labiano, S.; Perez-Gracia, J. L.; Martin-Algarra, S.; Alfaro, C.; Mazzolini, G.; Sarno, F.; Hidalgo, M.; Korman, A. J.; Jure-Kunkel, M.; Melero, I., Nivolumab and Urelumab Enhance Antitumor Activity of Human T Lymphocytes Engrafted in Rag2-/-IL2Rgammanull Immunodeficient Mice. Cancer Res 2015, 75 (17), 3466-78.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃ 一年有效。 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们
























