-
生物活性
Thedissociation equilibrium constant (KD) measured by surface plasmon resonance(SPR) for the binding of soluble TNFα to immobilized golimumab was 18 pM.[1]
-
体外研究
-
体内研究
-
激酶实验
Assay for the Binding of Anti-TNF-α Agents to Transmembrane TNF-α[2]
Mock-transfected and transmembrane TNF-α–transfectedJurkat cells were incubated with infliximab, etanercept, adalimumab, certolizumabpegol, golimumab, or the control antibody rituximab (all at 1μg/mL)for 30 minutes at 4 oC in phosphate-buffered saline containing 2%fetal bovine serum (fluorescence-activated cell sorting [FACS] buffer). Thecells were washed 3 times with an FACS buffer. The cells that were incubatedwith infliximab, etanercept, adalimumab, and golimumab were stained with afluorescein-conjugated goat F(ab’)2 fragment to human IgG Fc as a secondaryantibody for30 minutes at 4 oC. Cells that were incubated withcertolizumab pegol were incubated with an anti-PEG-biotin antibody for 30minutes at 4 oC and stained with avidin–fluorescein for 45 minutes at4 oC. Fluorescence intensities were measured using an FACSCalibur flowcytometer.

-
细胞实验
Cytotoxicity assays[1]
Human KYM-1D4 rhabdomyosarcoma cells ormurine WEHI-164 fibrosarcoma cells were seeded in RPMI 1640 (supplemented with2 mM glutamine and 10% fetal bovine serum) containing 2μg/mL ofactinomycin D and incubated at 37°C and 5% carbon dioxide for 4 h. Serialdilutions of each antibody were pre-incubated with human TNFa for 30 min. Thecells were then incubated for 16 h at 37°C.3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyl-tetrazolium bromide (MTT), at a finalconcentration of 0.5 mg/mL, was added and incubation continued for 3 h. Themedium was removed, and the insoluble MTT metabolic product was solubilized byadding 100 mL of dimethyl sulfoxide. Optical densities of 550–650 nm were usedas an indicator of cell viability.
-
动物实验
Tg197 transgenic mouse model[1]
Four-week-old, female, hemizygous miceobtained from the litters of homozygous Tg197 males bred with F1 (non-transgenic) females were assigned to one of five treatment groups (n = 10)so that the average body weight was similar across groups. Study groupsreceived a single intraperitoneal bolus of golimumab 1 mg/kg, golimumab 10mg/kg, infliximab 1 mg/kg, infliximab 10 mg/kg or vehicle (PBS). Mice wereweighed and assessed weekly for disease activity using a modified scoringsystem, whereby 0 = normal, 1 = edema or distortion of paw or ankle joints, 2= distortion of paw and ankle joints and 3 = ankylosis of wrist or anklejoints. The arthritic index was defined as the sum of scores from all fourpaws.
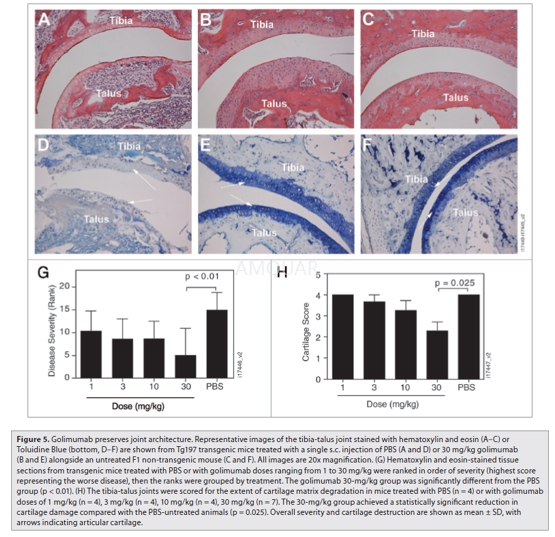
Histology was assessed in a secondexperiment in which five groups of Tg197 mice (n = 8) received a single s.c.injection of golimumab (1, 3, 10 or 30 mg/kg) or PBS. Four-week-oldnon-transgenic F1 females served as the control group. All mice wereeuthanized at day 23, rather than at the planned week 4 time point for ethicalreasons related to the severity of disease in the vehicle control group. Bothhind limbs were fixed intact by immersion in 10% neutral buffered formalin, decalcified,dehydrated and embedded in paraffin. Mid-sagittal sections (5 μm)were cut through the tibio-tarsal and metatarsal joints and stained withhematoxylin and eosin. Sections were evaluated in a blinded fashion and rankedin order of global disease severity (synovitis, cartilage destruction and boneerosion) with the highest score representing the worst disease. The blind wasbroken, the mice grouped according to treatment and the ranks from eachtreatment compiled. Evaluation of cartilage degradation was performed onsections stained with Toluidine Blue. Proteoglycan depletion and matrix erosionare associated with cartilage matrix destruction and are accompanied by a lossof Toluidine Blue staining. Cartilage destruction was scored from 0–4 using thesystem described by Douni and colleagues, whereby 0 = intact, 1 = minor(<10%), 2 = moderate (10–50%), 3 = high (50–80%), and 4 = severe (80–100%).Several tibio-talus joints could not be scored for cartilage damage due toinsufficient material or irregular staining in the tissue section (four eachfrom PBS and golimumab 1 mg/kg, 10 mg/kg; five from golimumab 3 mg/kg; and oneeach from golimumab 30 mg/kg and F1 non-transgenic controls).
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Shealy DJ, C. A., Staquet K, Baker A, Lacy ER, Johns L, Vafa O, Gunn G 3rd, Tam S, Sague S, Wang D, Brigham-Burke M, Dalmonte P, Emmell E, Pikounis B, Bugelski PJ, Zhou H, Scallon BJ, Giles-Komar J, Characterization of golimumab, a human monoclonal antibody specific for human tumor necrosis factor α. MAbs 2010, 2 (4), 428-39.
[2] Ueda, N.; Tsukamoto, H.; Mitoma, H.; Ayano, M.; Tanaka, A.; Ohta, S.; Inoue, Y.; Arinobu, Y.; Niiro, H.; Akashi, K.; Horiuchi, T., The cytotoxic effects of certolizumab pegol and golimumab mediated by transmembrane tumor necrosis factor alpha. Inflamm Bowel Dis 2013, 19 (6), 1224-31.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们


















