-
生物活性
Etanercept, a TNFα receptor Fc fusionprotein that binds to IgG1, is used for the treatment of rheumatic disease andpsoriasis.
FcRnbinding affinity (KD) is 1500 nM for etanercept.[1]
Inthe L929 murine assay system, etanercept has an inhibitory concentration of 90% (IC90) of 0.7ng/mL.[2]
-
体外研究
-
体内研究
-
激酶实验
In vitro human FcRn binding assay andquantification[1]
A label-free surface plasmonresonance-based assay was used to determine the kinetics (on and offrates) andaffinity (binding strength) of the interaction between the anti-TNFs and humanFcRn.
Human FcRn extracellular domain (IgGFc-binding domain) was expressed in CHO cells transiently transfected withhuman FcRn alpha chain and beta 2-microglobulin (B2M). The FcRn-B2Mheterodimer was prepared by affinity chromatography on a column of human IgG.The human FcRn extracellular domain was immobilized on the test chip by aminecoupling to a level of 267 RU (responseunits). Samples were passed over theFcRn-coated chip (30μL/min)in running buffer (0.02 M phosphate/0.15 M sodium chloride,pH6.0/0.05% v/v polysorbate P20) for 5 min at a range of concentrations (0 nM,21 nM, 42 nM, 84 nM, 168 nM, 335 nM and 670 nM)to determine the bindingon-rate; pH 6 buffer was used to allow optimum allowo ptimum binding. The FcRnbinding model represents the intracellular environment and endosomalinternalisation of IgG bound to the extra-celluar FcR domain, which is known tobe optimal at pH 6 and inactive at physiological pH. The off-rate was followedfor a further 5 min by running buffer alone over the chip. A zero controlsensorgram was run every second cycle in order to account for baseline driftand bulk buffer effects; a total of 30 such controls were tested and allproduced overlying sensorgrams close to baseline. The chip surface wasregenerated between cycles using 3 x 80 s pulses of regeneration buffer (200 nMsodium chloride/100 mM TRIS, pH 8.0). Data were normalized by subtracting blankflow cell data and mean zero control cycle data. The average dissociationconstant (KD) for each compound was calculated from a global fit of associationand dissociation kinetics measured over six concentrations (12 replicateexperiments).
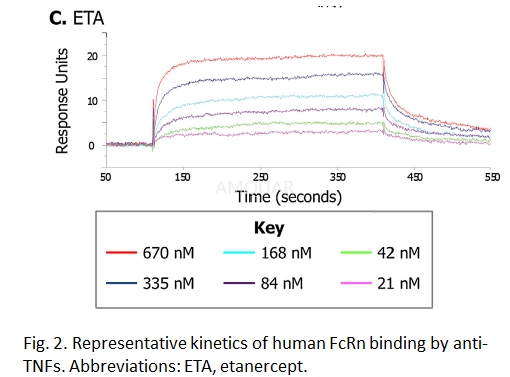
-
细胞实验
In vitro human FcRn transcytosis assay and quantification[1]
Madin Darby Canine Kidney (MDCK-II) cellstransfected with human FcRn and B2M were cultured in minimal essential medium(MEM) supplemented with 10% v/v fetalbovine serum (FBS), 2 mM l-glutamine, 1%w/v non-essential aminoacids, and 1% w/v sodium pyruvate (Invitrogen) at 37◦C/5% CO2.
The cells were cultured for 3 days in a24-well trans well plate, until an intact monolayer was formed, confirmed bymeasuring trans-epithelial resistances, which were at least 150Ωcm2.The cells were washed with pH 7.2 Hanks’ balancedsalt solution (HBSS), and biotinylated anti-TNFs were added to the apicalsurface in HBSS pH 5.9 with 1% v/v bovine serum albumin (BSA) (pH adjustedwith10 mM 4-morpholineethanesulfonic acid [MES]) at 10μg/mL. HBSS pH7.2 with 1% v/v BSA (buffered with 10 mM HEPES) was added to the basolateralside. The use of 1% v/v BSA in the apical and basolateral surfaces wasconsistent with the methods described in theliterature. Anti-TNF concentrationwas quantified in the basolateral supernatant after 4h’ incubation at 37◦C. The 4-h sampling time was optimal for the assay to establish the maximumsignal from the transcytosis assay without interfering with the integrity ofthe monolayer and was validated for spe-cific FcRn-dependent transport with theP146 control (FcRn binding abolished) antibody in all assays. The amount ofeach anti-TNF transcytosed was measured using a mesoscale discovery (MSD) electrochemiluminescentassay. The biotinylated anti-TNFs were captured on an MSD plate with an anti-humanIgG antibody (Jack-son Labs), then detected with a streptavidin sulphotagreagent (MSD). The electrochemiluminescent signal was determined using an MSDSector Imager 6000 plate reader. Levels of each test anti-TNF were determinedby comparison to a standard curve for each corresponding anti-TNF tested.Average (arithmetic mean) amount of anti-TNF transcytosed and the standarddeviation (SD), were calculated for each anti-TNF from 3 replicate experiments.
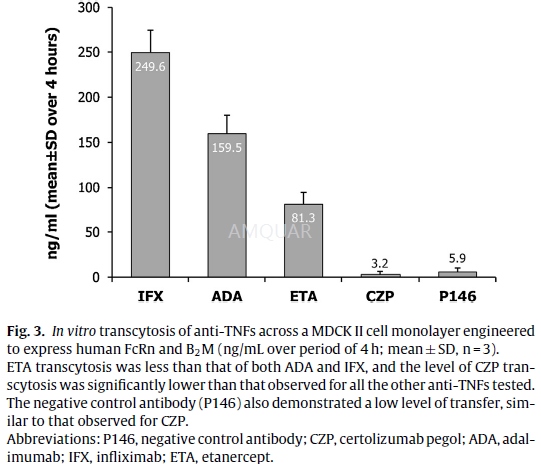
-
动物实验
Animals[3]
For this study, 40 male-adultSpraque-Dawley rats, weight ranging from 250g to 300g which have neverparticipated in any test before, were used.
Groups
Experiment animals were randomly dividedinto 4 groups, each of which has 10 rats.
Group 1 (Control group, C): No head traumawas applied to the rats in this group and they were not given any treatment.
Group 2 (Trauma + NS group, TP): Headtrauma was applied to the rats in this group and then they were given NS.
Group 3 (Trauma + Dexamethasone group, TD):First, head trauma was induced in the rats in this group, and thendexamethasone was given to them.
Group 4 (Trauma + Etanercept group, TE):Head trauma was induced in the rats in this group and etanercept was given tothem afterwards.
Anesthesiaand Creating Trauma
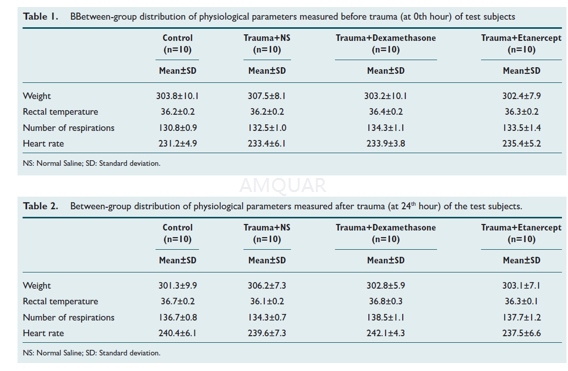
Before Anesthesia, all test subjects wereweighed and they were given 50 mg/kg ketamine hydrochloride by intraperitonealroute. After checking anesthetic depth by corneal reflex and tail pinch test,the physiological values such as breathing, pulse, rectal temperature of theanimals were recorded at 0th and 24th hour (Table 1, 2).
The animals were weltered in the groups inwhich trauma was to be induced. A skin incision was made along the mid-line ina way in which bregma and lambdoid suture could be seen. Periosteum wasseparated from the edges so that sutures could be seen totally from the frontto the back. A steel disc with diameter of 10 mm and thickness of 3 mm (Figure3a ve 4a) was placed into the midline between coronal and lambdoid sutures.Subsequently, the rats were placed in prone position on a sponge groundmeasuring 12x12x43 cm and the trauma tool was positioned. A 450g steel bar targetingthe animal’s head was dropped from 2 m height through a tube, with 19 mm insidediameter and 25 mm outside diameter. Rats whose respiration was stopped soonafter the trauma and whose pupils were dilated and who had seizure (respiratoryarrest, 5 rats; seizure, 3 rats) were resuscitated by performing CPR. Theresuscitation was continued until the sufficient respiration recovered. Theskin incisions were sutured with 2/0 silk suture. The animals whose respirationrecovered were taken to their cages. Two rats killed during the trauma werereplaced with the new ones, so the equal numbers in the groups were maintained.According to the treatment protocol in respective groups, the test subjectswere given dexamethasone or etanercept via intraperitoneal route in variousdoses soon after the resuscitation. At 24th hour, all animals were decapitated;their brain and brain stem were removed as a whole and were fixed by 10%formalin.
HistopathologicAssessment
Brain materials, after fixation for 48hours with 10% buffered formalin, were sliced in coronal plane, sampled as twofrom front to back and taken in tissue processing. Once they were dehydratedwith the formalin, alcohol and xylene successively, paraffin blocks wereprepared. After hydration and deparaf finization, hematoxylin eosinstain and immunohisto-chemical stains such as anti caspase-3, anti iBA-1, antiGFAP, and anti NeuN were applied to 5 mm cross-sections taken from paraffinblocks, as the manufacturer states. The preparates studied by Nikon Eclipse 80ilight microscope were transferred into the digital platform by Nikon DS-Fi1camera attachment. In histopathological assessment, existence and severity ofedema, and existence and severity of inflammation were used as variables. Theexistence of edema was assessed according to occurrence of microcystic areas byopening inside of the cells in parenchyma, however, the severity of edema wasassessed as 1+ when edema is less than 10% in a magnification of 20x inmicroscope, 2+ when it is between 10% and 50% and 3+ when it is over %50. Theexistence of inflammation was conducted by searching the existence of leukocytewith polymorphic nuclei, lymphocyte, plasma cell and eosinophils.
ImmunohistochemicalAssessment
After the most affected sides in terms ofcell damage were detected, preparates obtained as immunohistochemically weretaken into the digital platform by taking view from the most effected sidesthree at a time during the large magnification (x200). The number ofcytoplasmic cell GFAP, iBA, and Caspase 3 and the number of nuclear positivestained cell of NeuN was counted by means of digital program counter. Inimmunohistochemical assessment, astrocytes, microglial cells, severity anddamage occurring in neurons, and severity and existence of glial apoptosis weretaken as variables. The existence of glial apoptosis was conducted bysearching, with anti Caspase 3, the existence of glial cell that showscytoplasmic positivity. The damage occurring in astrocytes was detected bysearching, with anti GFAP (Glial fibrillary acidic protein), the existence ofcell that shows cytoplasmic positivity. The damage occurring in microglialcells was detected by searching, with anti Iba-1, existence of cell that showscytoplasmic positivity while the damage occurring in neurons was detected bysearching, with anti NeuN (Neuron-Specific Nuclear Protein), existence of cellthat shows nuclear positivity. The severity of the occurring damage in cellswas assessed as 1+ when the number of cells that shows nuclear and cytoplasmicpositivity was less than 10%, 2+ when it was between 10% and 50%, and 3+ whenit was over % 50.
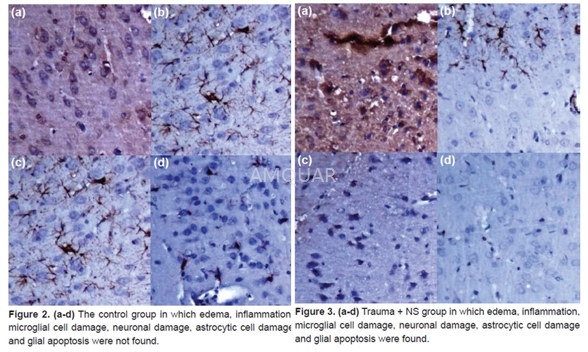

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Porter, C.; Armstrong-Fisher, S.; Kopotsha, T.; Smith, B.; Baker, T.; Kevorkian, L.; Nesbitt, A., Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): Consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transfer. J Reprod Immunol 2016, 116, 7-12.
[2] Nesbitt, A.; Fossati, G.; Bergin, M.; Stephens, P.; Stephens, S.; Foulkes, R.; Brown, D.; Robinson, M.; Bourne, T., Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis 2007, 13 (11), 1323-32.
[3] Aykanat, O.; Karakoyun, D. O.; Turkoglu, M. E.; Dinc, C., Anti-edematous, anti-inflammatory and neuroprotective effect of etanercept in acute stage in experimental head injury. Ulus Travma Acil Cerrahi Derg 2017, 23 (3), 173-180.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们





















