-
生物活性
Eculizumab, a humanized anti-complement C5monoclonal antibody for treatment of paroxysmal nocturnal hemoglobinuria (PNH)and atypical hemolytic uremic syndrome, blocks the terminal complement pathwayrequired for serum bactericidal activity (SBA).
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
In vitro MFS model[1]
Mouse hemi-diaphragms with phrenic nerveattached(or triangularis sterni muscle in some cases for illustrative NMJimmunohistology) were dissected and mounted in Ringer’s medium at roomtemperature (20–22oC). Untreated small control sections were removedfrom each muscle preparation prior to any incubations and snap frozen on dryice for subsequent baseline immunohistological analysis. Muscles were incubatedwith CGM3 (50μg/ml) for 2–2.5 h at 32oC, then for 30 min at 4oCand then equilibrated for 10 min at room temperature, rinsed in Ringer’s mediumand subsequently exposed to 33 or 40% NHS in Ringer’s medium for 1 h at roomtemperature. Eculizumab (100μg/ml) or the control mAb (100μg/ml) wasmixed with NHS 10 min prior to the incubation of the muscle preparation.
In vitro electrophysiological measurementsat the NMJ (see below) and scoring of muscle fibre twitching, a hallmark ofuncontrolled transmitter release at NMJs in this model, were performed beforeand after CGM3 incubation, and during the 1 h NHS incubation. Muscle stripswere frozen on dry ice and stored at -20oC, both before and afterincubation with NHS. NMJs and terminal motor axons were assessed for levels ofIgM, C3c, MAC, neurofilament and for perisynaptic Schwann cell (pSC) viability(see below).
Invitro electrophysiological analysis of NMJs and visual scoring of musclecontractility
Left and right hemi-diaphragms with theirphrenic nerve attached were dissected and pinned out in a silicone rubber-lineddish in 1.5 ml Ringer’s medium at room temperature. Intracellular recordings ofminiature endplate potentials (MEPPs, the postsynaptic events arising fromspontaneous release of single acetylcholine quanta from the presynaptic motornerve terminal) were made at NMJs at 20–22 oC. Muscle fibres wereimpaled near the NMJ with a 10–20MΩ glass micro-electrode filled with 3MKCl, connected to a GeneClamp 500B amplifier for amplifying and filtering (10kHz low-pass) of the signal. Signals were digitized, stored and analysed(off-line) using a Digidata 1322A interface, Clampex 9.2 and Clampfit 9.2programs, Mini Analysis 6.0 and routines programmed in Matlab.
The occurrence of spontaneous asynchronousfibre twitches that are induced by a high MEPP frequency resulting from CGM3/complement-mediated presynaptic damage was scored visually every 5 min. Thetissue scored 0 for no activity, 1 for the twitching of <10 fibres, 2 for asmall amount of twitching across the tissue, 3 for a moderate amount and 4 foran extensive amount. The average score during each measuring period wascalculated. The final result of CGM3/ complement-mediated presynaptic damage isa silencing of synaptic transmission at the NMJ. The number of ‘silent’ NMJs(i.e. without detectable MEPPs and occurrence of phrenic nervestimulationevoked muscle action potentials) was expressed as percentage of the totalnumber of NMJs sampled (8–41) in a muscle under each experimental condition.
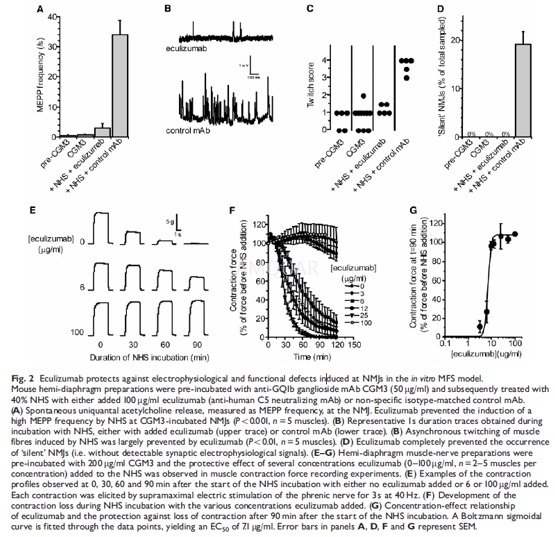
-
动物实验
Mice[1]
Male Balb/c mice (3–6 weeks old, 10–25 g)were obtained. In some muscle contraction experiments (see below) male andfemale GM2/GD2-synthase null-mutant mice were used at 12–22 weeks of age (19–37g).
Invivo MFS model
Balb/c mice (3–4 weeks old, 10–15 g) wereinjected intraperitoneally with 1.5 mg CGM3, followed 16 h later by an intraperitonealinjection of 0.5 ml 100% NHS. Mice were observed for a further 4–6 h andanalysed with whole-body plethysmography (see below) and grip-strength testing.The mice were then killed by CO2 asphyxiation and hemidiaphragmmuscle tissue was dissected and analysed in electrophysiological and musclecontraction experiments (see below) or further processed for immunohistologicalanalyses (see below). The effect of eculizumab on the in vivo symptoms and thesubsequently analysed electrophysiological, functional and histological lesionswas tested in this model by injecting 200μg eculizumabor control mAb in the tail vein, shortly before intraperitoneal NHS injection.Initial dose-range finding experiments indicated that eculizumab at doses of >50μg/mouse protected against neuropathy.
Plethysmography
Non-invasive whole-body plethysmography wasemployed to demonstrate changes in breathing parameters in in vivo experiments.Baseline data was collected prior to onset of experiment. Mice were injectedwith CGM3 followed by NHS with eculizumab or control mAb 16 h later (protocolas above) and monitored continuously for 6 h. Flow derived parameters of breathfrequency and tidal volume were collected from 25 accepted breaths and averagedover 1 h periods.
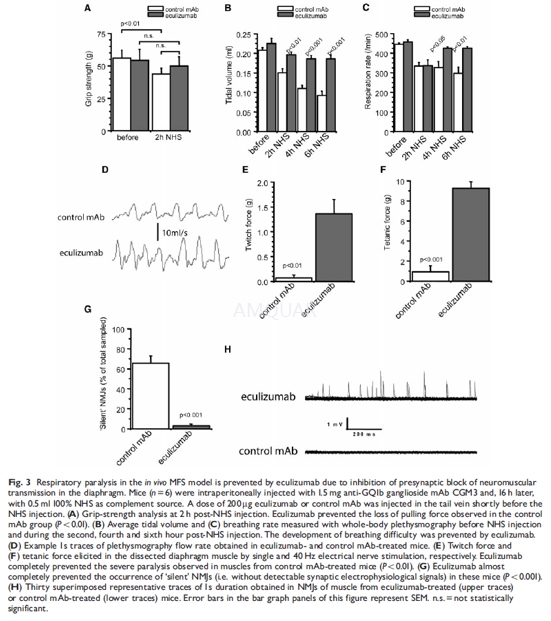
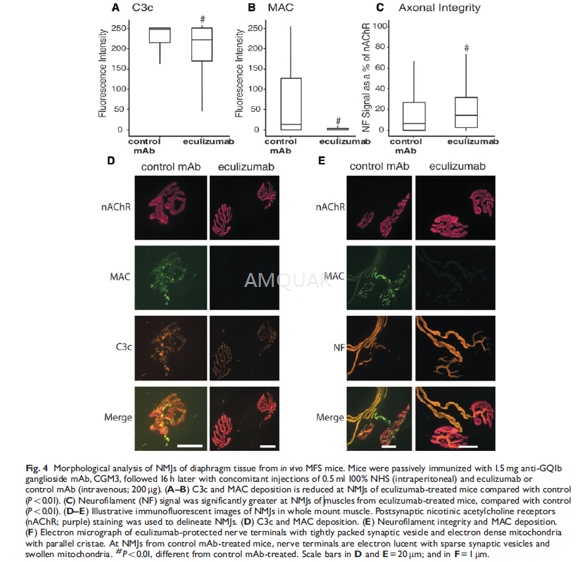
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Halstead, S. K.; Zitman, F. M. P.; Humphreys, P. D.; Greenshields, K.; Verschuuren, J. J.; Jacobs, B. C.; Rother, R. P.; Plomp, J. J.; Willison, H. J., Eculizumab prevents anti-ganglioside antibody-mediated neuropathy in a murine model. Brain 2008, 131 (5), 1197-1208.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们



















