-
生物活性
Denosumab, a human monoclonal antibodyagainst receptor activator of nuclear factor κ B ligand(RANKL), has become widely used as an anti-osteoclastic agent for osteoporosis.
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
β-Cell Histomorphometry and Immunostaining[1]
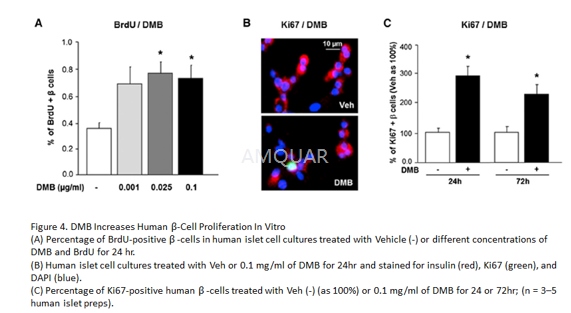
For in vitro studies, mouse and humanislets were trypsinized and cultured on glass coverslips in complete media for24 hr. Subsequently the cells were cultured in serum-free medium containingeither vehicle or species-compatible OPG-Fc, or Denosumab (DMB) at specificconcentrations, with BrdU (10 mg/ml) added in the last 24 hr of treatment,after which they were fixed in 2% paraformaldehyde for 30 min. b-cellproliferation was quantified using either BrdU or Ki-67 co-staining withinsulin. Human islet cells were costained with Ki67 (1:300) and NKX6.1 (1:300)and DAPI.
-
动物实验
Treatment in Mice[1]
C57BL/6 8- to 10-week-old or 1-year-oldmale mice were injected subcutaneously (sc) with saline or different doses(0.01–1.0 mg/g body weight) of mOPG-Fc, either daily for 7 days or everyalternate day for 30 days. In the MLDS model, 10-week-old C57BL/6 male miceinjected daily for 5 days with 50 mg/kg of STZ were administered vehicle ormOPG-Fc (1.0 mg/g body weight) daily for 16 days, with blood glucose measuredevery alternate day. 12- to 14-week-old male OPG-KO and WT littermates wereinfused with either saline or oPRL (0.175 mg/hr) for 7 days using Alzetmicro-osmotic pumps implanted sc between scapulae. Pancreata were harvested 6hr after an intraperitoneal BrdU (50 mg/g) injection. All animal studies wereperformed with the approval of, and in accordance with, guidelines establishedby the University of Pittsburgh, and the Icahn School of Medicine at MountSinai, and principles of laboratory animal care were followed.
HumanIslet Transplants
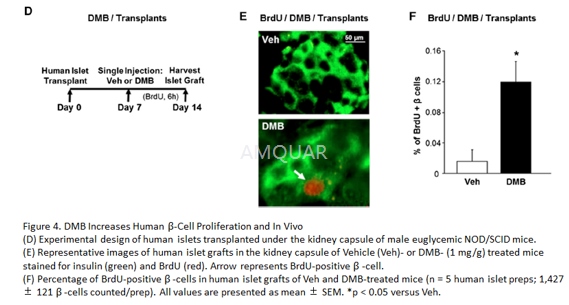
Two 8- to 12-week-old male euglycemicNOD/SCID mice were transplanted under the kidney capsule with 1,000 isletequivalents (IEQ; 1 IEQ = 125 mm diameter) of each human islet prep. Mice were injectedsc with vehicle or Denosumab (DMB) (1 mg/g body weight) at day 7post-transplant. On day 14, the kidney with the islet graft was harvested for β-cellproliferation and β-cell death analysis.
β-CellHistomorphometry and Immunostaining
Pancreata were weighed, fixed in Bouin’s,and paraffin embedded. Histomorphometry for β-cell masswas performed in a blinded way on 5–7 insulin-stained pancreatic sections peranimal separated by 50 mm each, using the Optimas software package. β-cellmass was quantified per animal as the ratio of the insulin-positive to totalpancreatic area, multiplied by the pancreas weight and averaged for all thesections/mouse. β-cell proliferation was quantified as percentage of BrdU-insulin orpHH3-insulin to total insulin-positive cells. Pancreatic sections were stainedwith antibodies against insulin (Dako) and BrdU (1:200 dilution) or pHH3(1:500), using an immunofluorescence secondary antibody (Williams et al.,2011). Mouse pancreatic sections were co-stained for BrdU and PDX1 (1:300), andDAPI. β-cell death was quantified as a percentage of TUNEL-insulin to totalinsulin- positive cells on pancreatic sections stained with antibodies againstinsulin and TUNEL (Promega). In human islet transplant studies, mice wereinjected with BrdU on day 14, and 6 hr later the kidney with the islet graftwas harvested, fixed in Bouins, and b-cell proliferation was analyzed on atleast 3–4 BrdU-insulin co-stained sections/islet graft andβ-celldeath on TUNEL-insulin co-stained sections.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Kondegowda, N. G.; Fenutria, R.; Pollack, I. R.; Orthofer, M.; Garcia-Ocana, A.; Penninger, J. M.; Vasavada, R. C., Osteoprotegerin and Denosumab Stimulate Human Beta Cell Proliferation through Inhibition of the Receptor Activator of NF-kappaB Ligand Pathway. Cell Metab 2015, 22 (1), 77-85.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们


















