细胞实验
Cells, antibodies and drugs[1]
NSCLC cell lines were cultured in RPMImedia in the presence of 10% fetal calf serum (FCS) with (SW-1573, H322 andH441) or without 2 mM L-glutamine (A549, H358 and H292) and incubated at 37 °Cin a humidified atmosphere with 5% CO2. A431 cells were cultured inDMEM high glucose in the presence of 10% FCS.
For monoclonal antibody treatments a totalconcentration of 20μg/mL was used unless otherwise indicated. Erlotinib andbafilomycin A1 were dissolved in dimethyl sulfoxide (DMSO), stored at −20 °Cand diluted in fresh medium for use. The final concentration of DMSO neverexceeded 0.1% v/v.
Flow cytometry
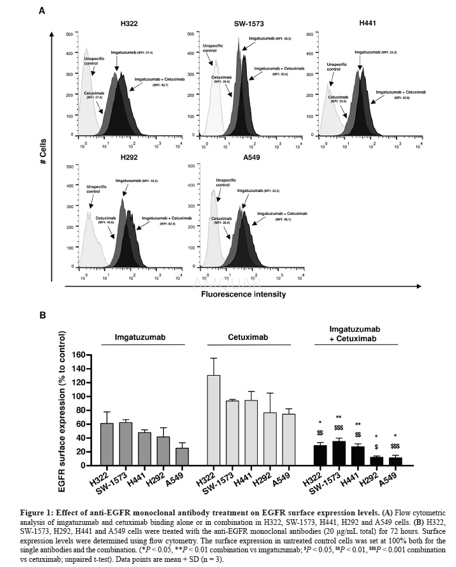
Analysis of EGFR expression in the cancercell lines was performed using flow cytometry. Cells were harvested in phosphate-bufferedsaline (PBS: 9.7mM Na2HPO4, 1.6mM KH2PO4,150mM NaCl, pH = 7.2) containing 2% FCS (FACS buffer) and left on ice prior toflow cytometry analysis. EGFR membrane expression levels were measured usingthe anti-EGFR human antibody imgatuzumab or cetuximab at final concentrationsof 20μg/mL in FACS medium. Bound antibody was detected using mouse anti-humanFITC diluted 1:50 in FACS medium.
To determine the effects of anti-EGFRmonoclonal antibody treatment on EGFR membrane levels, cells were treated withimgatuzumab (20μg/mL), cetuximab (20μg/mL) or the combination (10μg/mL each or20μg/mL each) for 24 and 72 hours. Cells were harvested in FACS buffer and lefton ice prior to flow cytometry analysis. To determine the internalization ofEGFR monoclonal antibodies and membranous turnover of EGFR in tumor cells,cells were stained on ice with the primary antibodies against EGFR (20μg/mLfinal concentration). After staining with the primary antibody; 1) cells werewashed with ice-cold FACS buffer and incubated with the secondary antibody for1 hour at 4 ˚C to measure surface expression. 2) Cells were washed withice-cold FACS buffer, incubated in culture medium at 37 ˚C for 1, 2, or 4 hoursand subsequently incubated with the secondary antibody for 1 hour at 4˚C tomeasure non-internalized EGFR-antibody complexes since the secondary antibodiesonly bind to surface bound EGFR antibody. The lower the amount ofnon-internalized EGFR-antibody complexes, the more internalization ofEGFR-antibody complexes. 3) Cells were washed with ice-cold FACS buffer,incubated in culture medium at 37 ˚C for 1, 2, or 4 hours and subsequently incubated with theprimary antibody, followed by the secondary antibody to measurenon-internalized, reappeared receptors and possible de novo synthesis ofreceptors. Duplicate samples were measured for each treatment condition, andcorrected for background fluorescence and unspecific binding of the secondaryantibody. Measurement was performed on a BD FACSCalibur or BD Accuri C6. Dataanalysis was performed with FlowJo v10 and surface receptor expression wasexpressed as mean fluorescent intensity (MFI).
Growth inhibition and migration assays
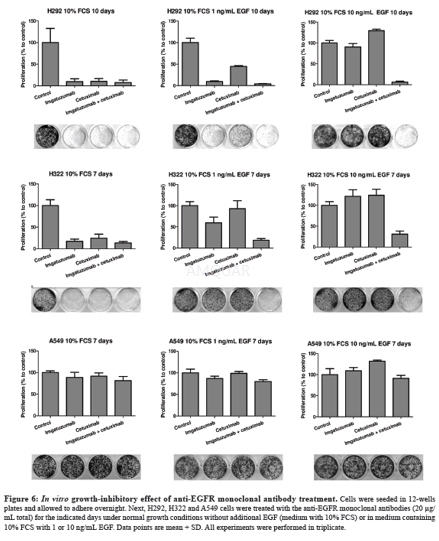
For the proliferation assays, SW-1573 (2000cells/ well), H292 (2000 cells/well), H322 (8000 cells/well), A549 cells (2000cells/well) and H358 (2000 cells/well) were seeded in 12 wells plates. Cellswere allowed to adhere overnight. Subsequently, cells were treated with theindicated anti-EGFR monoclonal antibodies (20μg/mL total) in complete mediumcontaining 10% FCS with or without the addition of 1 or 10ng/mL EGF andincubated for the indicated time period at 37 °C.
For the wound healing assays, H292 and A549cells (both 150,000 cells/well) were seeded in 24 wells plates. After 24 hourscells were serum starved overnight. Next, a scratch was made in the monolayerwith a pipette tip, wells were washed with PBS, and cells incubated in serumfree medium containing 10ng/mL EGF with or without the anti-EGFR monoclonalantibodies at 37 °C. Scratch area was measured when the scratch was made andafter 16 hours for H292 or 24 hours for A549 using Image J software analysis(1.47v).
ADCC assays
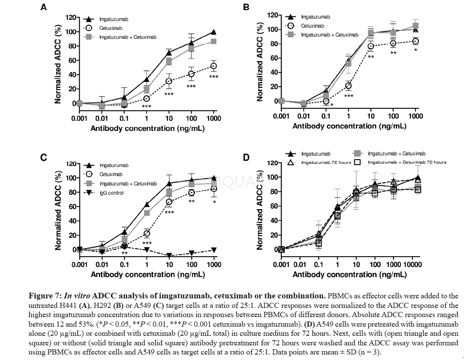
ADCC was measured using the LactoseDehydrogenase Cytotoxicity Detection Kit according to standard protocol. Briefly, humanPBMCs were prepared from healthy blood donors by Ficoll gradientcentrifugation. PBMCs were washed in PBS and resuspended in Aim V medium. Serialdilutions of either imgatuzumab or cetuximab were added to each well of a96-well plate. The final concentration of the monoclonal antibody combinationwas the same as in the single monoclonal antibody experiments. ADCC wasconducted using an effector:target (E:T) cell ratio of 25:1 and 4 hoursincubation at 37 °C. All assays were conducted in triplicate.
CDC assays
CDC was measured using the LactoseDehydrogenase Cytotoxicity Detection Kit. Anti-EGFR monoclonal antibodies (10μg/mL total) were added to 25.000 tumors cells in a 96-well plate. CDC wasconducted using a final concentration of 5% freshly drawn human serum and 4hours incubation at 37 °C. Antibody-dependent cytotoxicity without serum wasnot observed. All assays were conducted in triplicate.
参考文献
[1] Kol A, T. v. S. A., Pool M, Gerdes C, de Vries E, de Jong S, ADCC responses and blocking of EGFR-mediated signaling and cell growth by combining the anti-EGFR antibodies imgatuzumab and cetuximab in NSCLC cells. Oncotarget 2017.
[2] Zeng, L.; Beggs, R. R.; Cooper, T. S.; Weaver, A. N.; Yang, E. S., Combining Chk1/2 Inhibition with Cetuximab and Radiation Enhances In Vitro and In Vivo Cytotoxicity in Head and Neck Squamous Cell Carcinoma. Mol Cancer Ther 2017, 16 (4), 591-600.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度