-
生物活性
Certolizumabpegol binds to TNF with a KD of 89.3 pmol/L.[1]
Inthe L929 murine assay system, certolizumab pegol has an inhibitory concentrationof 90 % (IC90) of 3ng/mL.[2]
-
体外研究
-
体内研究
-
激酶实验
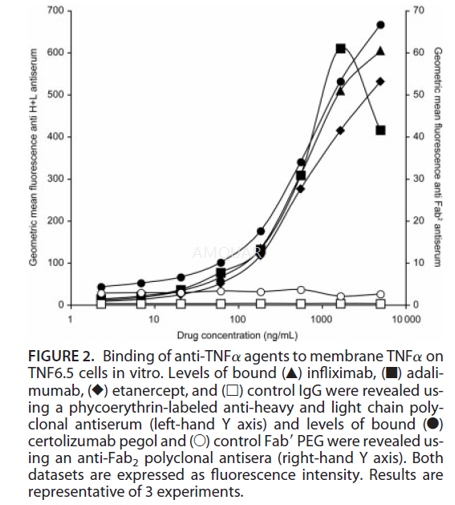
Binding of Anti-TNFα Agents to Membrane TNFα[2]
The ability of the anti-TNFα agents to bindmTNFα was assessed by fluorescence-activated cell sorter (FACS) analysis.TNF6.5 cells, which express mTNFα, were washed to remove any sTNFα from theculture supernatant and resuspended at 1.25 x 105 cells/mL of FACSbuffer (Dulbecco’s phosphate-buffered saline without Ca2+ and Mg2+ 5% [v/v] FCS, sodium azide 0.5% [w/v]]) in the presence of the anti-TNFα agents.The cells were left at 4oC for 30 minutes, then washed twice in FACSbuffer, incubated with phycoerythrin-labeled secondary antibody (100μLof 1/250 dilution) at 4oC for 30 minutes, then washed again before finalresuspension in FACS buffer and analysis by flow cytometry. An anti-Fab2 secondlayer polyclonal antiserum was used to reveal specific binding ofcertolizumab pegol, while an anti-H_L polyclonal antiserum was used to labelthe other 3 anti-TNFα agents (which are all based on human IgG1).
-
细胞实验
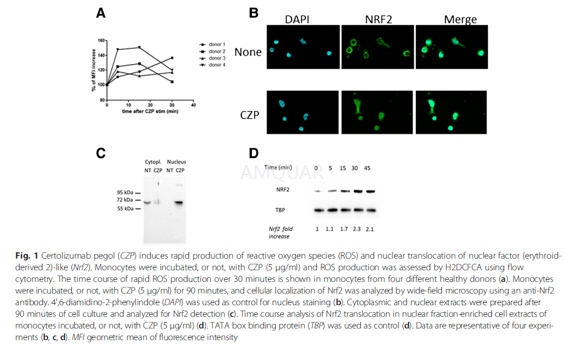
ROS measurements[3]
For intracellular ROS measurement, we usedthe H2DCFDA compound. Monocytes (4 × 105 cells) were grownin a 24 well-plate in 0.5 ml Macrophage SFM medium. All treatment conditionswere performed in duplicate. Monocytes were incubated, or not, for 5, 15 and 30minutes with 5μg/ml of certolizumab pegol(CZP). Then, culture medium wasdiscarded and replaced by PBS containing 2μM H2DCFDA. Monocytes wereincubated for 30 minutes and then detached from the well in 300μl of PBS 5 mMEDTA at 4 °C. The level of fluorescence (geometric mean) was measured by flowcytometry on a FACSCalibur cytometer.
In another experiment, monocytes wereincubated, or not, for 1 h with 5 μg/ml of CZP, washed three times with mediumto remove unfixed CZP, and then incubated for 18 h in medium containing LPS.The ROS production was then measured. All cytometry data were analyzed byFlowJo.
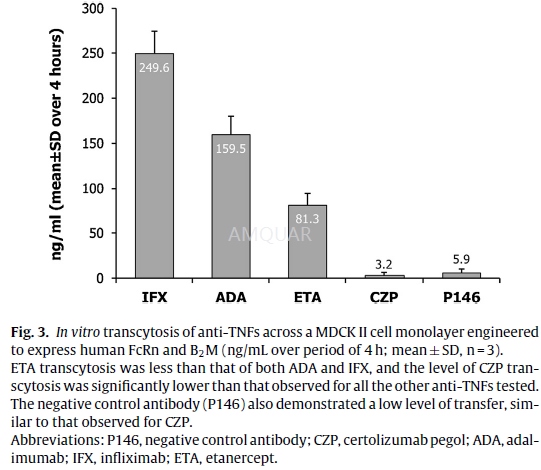
In vitro human FcRn transcytosis assay andquantification[4]
Madin Darby Canine Kidney (MDCK-II) cellstransfected with human FcRn and B2M were cultured in minimal essential medium(MEM) supplemented with 10% v/v fetalbovine serum (FBS), 2 mM l-glutamine, 1%w/v non-essential aminoacids, and 1% w/v sodium pyruvate (Invitrogen) at 37◦C/5% CO2.
The cells were cultured for 3 days in a24-well trans well plate, until an intact monolayer was formed, confirmed bymeasuring trans-epithelial resistances, which were at least 150Ωcm2.The cells were washed with pH 7.2 Hanks’ balancedsalt solution (HBSS), and biotinylated anti-TNFs were added to the apicalsurface in HBSS pH 5.9 with 1% v/v bovine serum albumin (BSA) (pH adjustedwith10 mM 4-morpholineethanesulfonic acid [MES]) at 10μg/mL. HBSS pH7.2 with 1% v/v BSA (buffered with 10 mM HEPES) was added to the basolateralside. The use of 1% v/v BSA in the apical and basolateral surfaces wasconsistent with the methods described in theliterature. Anti-TNF concentrationwas quantified in the basolateral supernatant after 4h’ incubation at 37◦C. The 4-h sampling time was optimal for the assay to establish the maximumsignal from the transcytosis assay without interfering with the integrity ofthe monolayer and was validated for spe-cific FcRn-dependent transport with theP146 control (FcRn binding abolished) antibody in all assays. The amount ofeach anti-TNF transcytosed was measured using a mesoscale discovery (MSD) electrochemiluminescentassay. The biotinylated anti-TNFs were captured on an MSD plate with an anti-humanIgG antibody (Jack-son Labs), then detected with a streptavidin sulphotagreagent (MSD). The electrochemiluminescent signal was determined using an MSDSector Imager 6000 plate reader. Levels of each test anti-TNF were determinedby comparison to a standard curve for each corresponding anti-TNF tested.Average (arithmetic mean) amount of anti-TNF transcytosed and the standarddeviation (SD), were calculated for each anti-TNF from 3 replicate experiments.
-
动物实验
Animals[5]
Male DBA/1 mice were housed in cages in anenvironmentally controlled room (temperature21–23°C and relativehumidity 38–50%) on a 12-h light/dark cycle. Animals had access to RM1 foodpellets and water ad libitum. Prior to the start of the experiment, all animalswere weighed and mice were divided into groups with similar starting weights.
Generationof arthritis disease model
Collagen-induced arthritis was induced.Chick sternal type II collagen (CII) was dissolved in 0.1M acetic acid at2mg/mL and constantly rolled overnight in the dark at 4 °C. The next day (day0) the CII solution was mixed with an equal volume of Complete Freund'sAdjuvant(CFA), containing 1mg/mL heat killed Mycobacterium tuberculosis, to give a 1mg/mL solution. The solution was mixed with a 5 mL syringe by repeatedlydrawing up and down to produce an emulsion. The correct consistency wasachieved when a drop of emulsion could be placed in water without sinking ordissipating. DBA/1 mice were anaesthetized using isoflurane and injectedintradermally at the base of the tail with 0.1 mL (100μg CII) ofemulsion. Each mouse was checked 30 min after this procedure. Fourteen dayspost-sensitization the mice were given a booster injection of CII following thesame method but using Incomplete Freund's Adjuvant instead of CFA. From day 14after the booster injection, animals were checked daily for signs of arthritis.When signs were seen, animals were weighed, disease scored on a 0–3 scale (asoutlined below), and distress scored daily.
Arthritisseverity scores
The severity of arthritis in the mice wasscored based on the level of inflammation in the paw and was recorded as 1 of 4grades: 0, normal paw; 1, wrist/ankle inflamed; 2, wrist/ankle and padinflamed; and 3,wrist/ankle, pad, and digits inflamed.
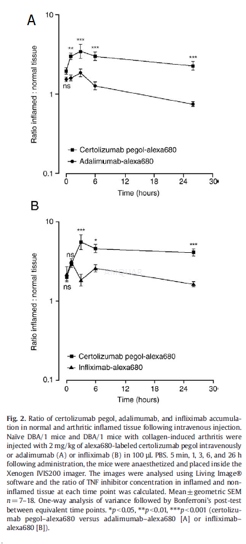
Administration of certolizumabpegol–alexa680, adalimumab–alexa680, and infliximab–alexa680
DBA/1 mice showing signs of arthritis weredivided into groups with similar disease scores. Arthritic mice and naïve DBA/1mice were administered 2 mg/kg protein of alexa680-labeled certolizumab pegolor adalimumab intravenously, and in a separate experiment, certolizumab pegolor infliximab intravenously in 100 μL PBS.
Biofluorescenceimaging
Mice were anaesthetized with gaseous 5%isoflurane in O2 and placed inside the Xenogen IVIS200 imager at 5min, 1, 3, 6, and 26 h after injection of alexa680-labeled certolizumab pegol,adalimumab, or infliximab. The animals were placed in a sternal recumbentposition and imaged from the dorsal aspect. The excitation filter passband was615–665 nm and the emission filter passband was 695–770 nm. Theacquisition time was 2 s, aperture f/stop 2, with 4×4 pixel binning. Directlyafter the 26-h time point, blood samples were taken via cardiac puncture andthe mice were killed.
Quantificationof certolizumab pegol–alexa680, adalimumab–alexa680, and infliximab–alexa680 inserum
Blood samples (50μL) were takenfrom the tail vein immediately prior to each imaging event. Serum was collectedfollowing centrifugation at 10,000 ×g for 5 min. Samples for analysis werediluted 1/10 in PBS and 100μL placed in a 96-well black plate for imaging. Theconcentration of alexa680-labeled certolizumab pegol, adalimumab, or infliximabin each sample was quantified using the XenogenIVIS200 imager. It was thencalibrated against a standard curve of alexa680-labeled certolizumab pegol,adalimumab, or infliximab (2.5–0.078 μg/mL) prepared in 10% normal mouse serum in PBS. GraphPadPrism® software was used to fit a 4-point logistic curve to thealexa680-labeled certolizumab pegol, adalimumab, and infliximab fluorescenceefficiency standard curves and the concentration of alexa680- labeledcertolizumab pegol, adalimumab, r infliximab in each sample was subsequentlydetermined.
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Pasut, G., Pegylation of Biological Molecules and Potential Benefits: Pharmacological Properties of Certolizumab Pegol. BioDrugs 2014, 28 (S1), 15-23.
[2] Nesbitt, A.; Fossati, G.; Bergin, M.; Stephens, P.; Stephens, S.; Foulkes, R.; Brown, D.; Robinson, M.; Bourne, T., Mechanism of action of certolizumab pegol (CDP870): in vitro comparison with other anti-tumor necrosis factor alpha agents. Inflamm Bowel Dis 2007, 13 (11), 1323-32.
[3] Boyer, J. F.; Baron, M.; Constantin, A.; Degboe, Y.; Cantagrel, A.; Davignon, J. L., Anti-TNF certolizumab pegol induces antioxidant response in human monocytes via reverse signaling. Arthritis Res Ther 2016, 18, 56.
[4] Porter, C.; Armstrong-Fisher, S.; Kopotsha, T.; Smith, B.; Baker, T.; Kevorkian, L.; Nesbitt, A., Certolizumab pegol does not bind the neonatal Fc receptor (FcRn): Consequences for FcRn-mediated in vitro transcytosis and ex vivo human placental transfer. J Reprod Immunol 2016, 116, 7-12.
[5] Palframan, R.; Airey, M.; Moore, A.; Vugler, A.; Nesbitt, A., Use of biofluorescence imaging to compare the distribution of certolizumab pegol, adalimumab, and infliximab in the inflamed paws of mice with collagen-induced arthritis. J Immunol Methods 2009, 348 (1-2), 36-41.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们




















