-
生物活性
Canakinumab,a high-affinity human monoclonal anti IL-1β antibody, neutralizingIL-1β-mediated pathways, is approved for treatment of systemic juvenileidiopathic arthritis (SJIA).
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
Treatments[1]
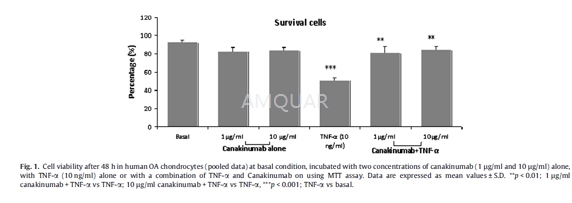
The first passage chondrocytes were seededin 24-well plates at a starting density of 4 x 104 cells/well andoverlaid with 2 ml of medium with phenol red containing 10% fetal calf serum, 200U/ml penicillin, 200 U/ml streptomycin, and 2 mM glutamine until they becameconfluent. The cells were then incubated with two concentrations (1μg/mland 10μg/ml) of canakinumab (ACZ885) alone or in combination with TNF-a(recombinant human TNF-α) at a concentration of 10 ng/ml for 48 h. After 24 h, for everysample 1 ml of the medium was collected and stored at -80 oC todetermine the concentration of IL-1β through an enzyme-linkedimmune-sorbent assay (ELISA) kit. At the end of the treatment, the remainingmedia were removed and immediately detected for NO release using Griess’s assayand then stored at -80 oC for detection of PG. The chondrocytes wereimmediately processed for cell viability, immunocytochemistry, detection ofMMPs and Annexin V (AnV)-fluorescein isothiocyanate (FITC)/propidium iodide(PI) assay, and morphological assessment using TEM.
Cellviability
Before and after each experimentalcondition, we evaluated cell viability by MTT (3,[4,4-dimethythiazol-2-yl]-2,5-diphenyl-tetrazolium bromide) assay based on the ability ofthe mitochondria of viable cells to convert soluble MTT into an insolublepurple formazan reaction product. MTT (5 mg/ml in DMEM without phenol red) wasadded to cells in tissue culture and incubated for 4 h. The media were then discarded,and 0.2 ml of dimethyl sulfoxide (DMSO) was added to each well to solubilizethe formazan crystals that had formed. The absorbance was measured at 570 nmusing a microplate reader. The percentage of cell survival was calculated as follows:
The experiments were carried out onpre-confluent cell cultures to prevent contact inhibition from influencing theresults. Cellular viability was assayed 48 h from the beginning of treatment.Results were expressed as optical density (OD) units per 104 adherentcells.
-
动物实验
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Cheleschi, S.; Cantarini, L.; Pascarelli, N. A.; Collodel, G.; Lucherini, O. M.; Galeazzi, M.; Fioravanti, A., Possible chondroprotective effect of canakinumab: an in vitro study on human osteoarthritic chondrocytes. Cytokine 2015, 71 (2), 165-72.
分子式
|
分子量
|
CAS号
|
储存方式
|
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们

















