-
生物活性
Brentuximab vedotin (SGN-35), aCD30-directed antibody-drug conjugate composed of an anti-CD30 monoclonalantibody linked to the microtubule-disrupting agent, is approved for treatingcertain patients with CD30-expressing hematologic malignancies.
-
体外研究
-
体内研究
-
激酶实验
-
细胞实验
Cells and reagents[1]
The CD30+ cHL cell lines L540,L428, KM-H2 and L1236, the CD30– cHL cell line HD-MyZ and the acutemyeloid (eosinophil) leukemia line EOL-1 were purchased from DSMZ. The mastcell line HMC-1 was from Dr. J. Butterfield (Mayo Clinic, Rochester MN). Cellswere cultivated in RPMI- 1640 or IMDM (HMC-1) containing 10% FCS, penicillin,streptomycin and 50 μM β-mercaptoethanol. EV-depleted FCS was generated by overnightcentrifugation at 100000 × g.
Flow cytometry
HMC 1.2 or EoL-1 cells (5 × 105/ml)were incubated for 1 h on ice with sCD30, ultracentrifugation-enrichedCD30-containing EVs or HBSS buffer alone. After washing with PBS containing 1%albumin and 0.1% sodium azide, cells were incubated for another 30 min on icewith FITC-coupled SGN-35 (0.1 μg/ml). After washing in the above PBS, cellswere evaluated by flow cytometry. Vesicles alone were incubated overnight withpolybead carboxylate microspheres (4.5 μm). The beads were blocked with 1% BSA(v/w) in PBS. Then, aliquots were incubated with unlabeled orfluorescence-labeled antibodies. Aliquots with unlabeled antibody were in a secondstep labeled with fluorescence-labeled anti-mouse IgG. Beads were evaluated byflow cytometry.
Toxicity of SGN-35
Cells were incubated for 96 h with sCD30,CD30EV and SGN-35 as indicated. Then, cells were washed and incubated for 15min at room temperature in annexin V binding buffer containing 0.8 μg/mlannexin V and 0.5 μg/ ml propidium iodide. After washing, cells were evaluatedby flow cytometry.
Western blot
Whole vesicle lysates generated after 2 minboiling with SDS sample buffer and cell-free supernatants were developed inSDS-PAGE and transferred to nitrocellulose. Membranes were blocked with PBScontaining 5% (w/v) fat-free dry milk and then incubated at 4°C with ADAM10 orBiotin-coupled SGN-35 mAbs (1 μg/mL each) and finally stained with peroxidase-coupledgoat anti-mouse IgG or streptavidin, respectively. ECL-reagent was used assubstrate.
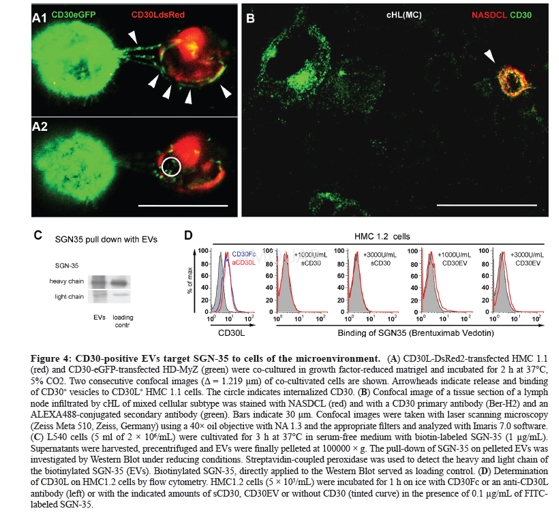
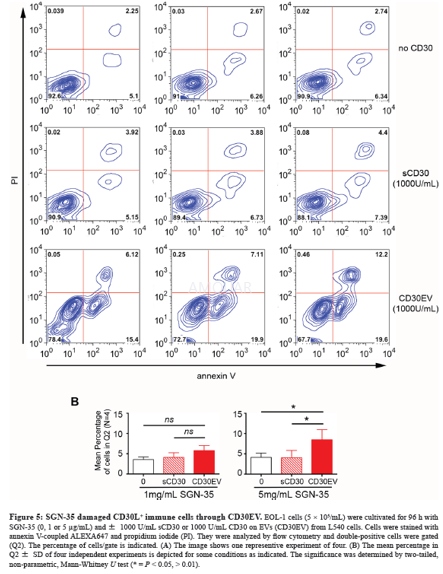
-
动物实验
L540cy tumour model[2]
Compounds tested include adriamycin (0.75mg/kg, q4dx3, i.v.), bleomycin (6 mg/kg, q4dx3, i.p.), vinblastine (0.01 mg/kg,q4dx3, i.p.), dacarbazine, gemcitabine (120 mg/kg, q4dx3, i.p.;) or vinorelbine(4 mg/kg, q5dx3, i.p.). The L540cy tumour model was build. To determine the maximumtolerated dose (MTD) of ABVD and gemcitabine, body weights of severe combinedimmunodeficient (SCID) mice were assessed daily after treatment with increasingamounts of drugs. The criteria defining the MTD were ≥20% decrease in body weights or other signsof morbidity during the entire treatment followed by a 2-week recovery period. Tumourquadrupling or tripling times were chosen as timeto- endpoint (TTE), which wasdetermined by using the nonlinear regression analysis for exponential growth ofeach individual tumour growth data set from each experimental animal. Thetumour quadrupling time was calculated based on the tumour volume at thebeginning of treatment. Animals that did not reach this endpoint were assigneda TTE-value equal to the last day of the study. %TGD (tumour growth delay)reflects the delay in reaching TTE relative to controltreated tumours, whichwas determined by using the formula: %TGD = [(T)C)/C] x 100, where T and C arethe median times in days for treated and control groups, to reach TTE, usingthe start of treatment as day 1. Statistical analysis and graphic presentationswere conducted using Prism (Graph- Pad) software for Windows 3.03 software.Tumour growth curves show group mean tumour volumes as a function of time. Datashown are from one representative of two independent experiments. The Logranktest was used to analyse the significance of the differences between TTE of treatedand control tumour groups, with differences deemed significant (*) at 0.01 ≤ P ≤ 0.05, and highlysignificant (**) at P ≤ 0.01.
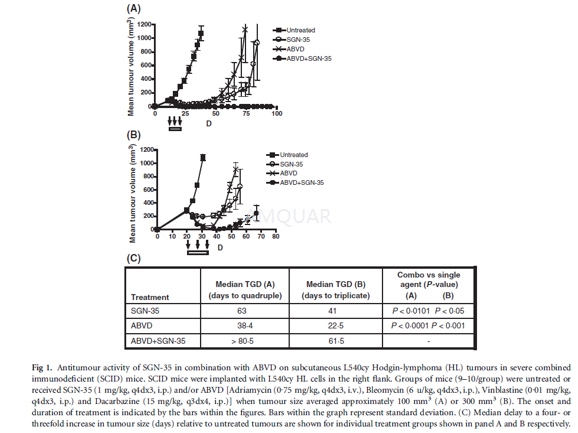
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Hansen HP, T. A., Dams M, Zigrino P, Moss M, Tator M, Schön G, Grenzi PC, Bachurski D, Aquino B, Dürkop H, Reiners KS, von Bergwelt-Baildon M, Hallek M, Grötzinger J, Engert A, Paes Leme AF, Pogge von Strandmann E, CD30 on extracellular vesicles from malignant Hodgkin cells supports damaging of CD30 ligand-expressing bystander cells with Brentuximab-Vedotin, in vitro. Oncotarget 2016, 7 (21), 30523-35.
[2] Oflazoglu, E.; Kissler, K. M.; Sievers, E. L.; Grewal, I. S.; Gerber, H. P., Combination of the anti-CD30-auristatin-E antibody-drug conjugate (SGN-35) with chemotherapy improves antitumour activity in Hodgkin lymphoma. Br J Haematol 2008, 142 (1), 69-73.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们



















