-
生物活性
Bevacizumab(Avastin), an antiangiogenic anti-VEGF-R monoclonal antibody specifically bindsto all VEGF-A isoforms with high affinity, has a rapid impact on tumor-relatedbrain edema in recurrent GB. Bevacizumabsuppresses GCSCs survival with an IC50 of ~ 6.5μg/ml.[1]
-
体外研究
Bevacizumabsuppresses GCSCs survival and enhanced the levels of apoptosis, p53 andcleaved-caspase3 along with a decrease in free VEGFA levels and ERKsactivation.[1]
-
体内研究
-
激酶实验
-
细胞实验
Primary culture of GCSCs[1]
Central zone of samples were freshlysubjected, washed with phosphate- buffered saline (PBS), minced andenzymatically dissociated via Trypsin, 0.25% (1×) with EDTA anddeoxyribonuclease I. And equal volume of soybean was used to stop the trypsinreaction. After centrifuging, the pellet was added RBC lysis buffer to removethe red blood cells. Afterward, the suspension was centrifuged and then thepellet was added Dulbecco's Modified Eagle Medium/nutrient mixture F-12(DMEM/F-12). The suspension was filtered through a 70-μmcell strainer. Singlecells were plated at density of 5 × 104 cells/ml in appropriate cellculture flasks consisting of serum free medium supplemented with B27 (5×)(Invitrogen, USA) at a 5:1 ratio, 20ng/ml human Epidermal Growth Factor (EGF),20ng/ml basic Fibroblast Growth Factor (b-FGF), 2μg/ml heparin and 1%antibiotic/antimycotic. Then the cultures were incubated at 37 °C in ahumidified chamber with 5% CO2. The GCSCs-derived spheres were replatedwhen the average size of them became 200 μm in diameter within one week. TheSpheres at passage five were used for the subsequent experiments.
Treatmentswith the Drugs
The GCSCs isolated from T2 at 5th passageshaving a purity of N95% based on the assessment of CD133 and CD15 expressions wereharvested and seeded in 24-well plates containing DMEM-F12 at a density of 2.5× 104 cells/well, overnight. The cells were treated with variousconcentrations (1, 3, 10, 30, 100 μg/ml) of bevacizumab for 48 h. Alltreatments were done in quadruplicate. After finding of halfmaximal inhibitoryconcentration (IC50) of bevacizumab, the cells incubated with bevacizumab atIC50 in the absence or presence of rolipram (103μM). Human Immunoglobulin G1(IgG1) at the same volume was used instead bevacizumab as control.
MTTAssay
At days 0, 2, 4, 6 and 8 of primaryculture, as well as at the end of treat- ment period, the cells were incubatedwith 5 mg/ml mehtylthiotetrazole (MTT) solution for 4 h. After centrifuging,the medium was carefully discarded and Dimethyl sulfoxide (DMSO) was added tosamples. Optical density (OD) was read at 570 nm by microplate reader. Theaverage of qua- druplicate absorbance readings values for each experimentalcondition was measured, separately.
HumanVEGFA ELISA
Human VEGF DuoSet ELISA kit was utilized tomeasure secreted VEGFA levels in culture medium at 48 h after treatments,according to the manufacturer's instructions. The absorbance at 450 nm wasevaluated by a microplate reader and soluble VEGFA concentrations werecalculated based on a standard curve.
CyclicAMP measurement
The intracellular levels of cyclic AMP wereassessed by Correlate-EIA™ Cyclic AMP Enzyme Immunoassay Kit according to the manufacturer's instructions.Briefly, At 48 h after treatments, T2-derived GCSCs collected, lysed in 0.1 MHCl solution and removed by centrifugation at N600 ×g for 10min. Assayprocedure running was initiated when lysates were resuspended in cAMP assaybuffer At termination, all reactions were laid off. Immediately, absorbance at405 nm was read by amicroplate reader, and cAMP concentrations were calculatedbased on a standard curve.
TUNELStaining
Terminal deoxynucleotidyl transferase dUTPnick end labeling (TUNEL) staining was carried out according to manufacturer'sinstructions. Briefly, the cells were fixed in fresh 4% paraformaldehyde atroom temperature for 60 min and then rinsed with PBS twice and incubated with0.1% Triton X-100 solution at 8 °C for 2 min. After washing, the cells wereincubated with 50 μl TUNEL reaction mixture at 37oC in a humidified chamber for60 min. The cells were counterstained with propidium iodide (PI) and visualizedusing Fluorescence inverted microscope (Olympus IX71). Apoptosis value wassemi-quantified by calculating TUNEL-positive cells number of each fieldrelative to PI-positive cells number (total cells) of the same field usingImage J software.

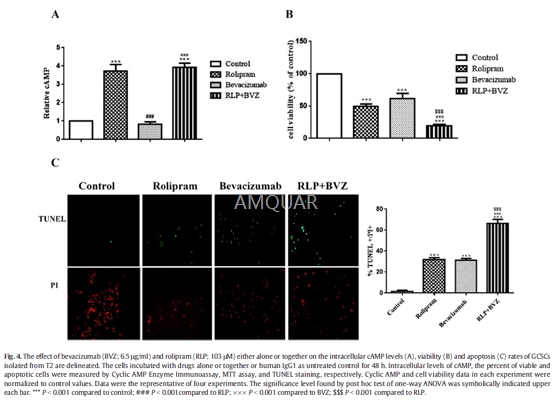
-
动物实验
Animals[2]
Male, 4- or 5-week-old BALB-nu/nu mice andmale, 6- week-old C.B-17/Icr-SCID Jcl severe combined immune-deficient (SCID)mice were purchased. All animals were housed in a specific pathogen–freeenvironment under controlled conditions (temperature, 20-26˚C; humidity,35-75%; light/dark cycle, 12/12 h) and were allowed to acclimatize and recover fromshipping-related stress for more than 6 days prior to the study. Chlorinatedwater and irradiated food were provided ad libitum. The health of the mice wasmonitored by daily observation.
Invivo tumor growth inhibition studies
Each BALB-nu/nu mouse was inoculatedsubcutaneously into the right flank with either A549 (5×106 cells)or NCI-H292 (5×106 cells). Each SCID mouse was inoculatedsubcutaneously into the right flank with NCI-H2228 (1×107 cells).Several weeks after tumor inoculation, the mice were randomly allocated tocontrol and induction treatment groups (day 1). As the induction treatment inthe A549 and NCI-H292 xenograft models, HuIgG or bevacizumab (5 mg/kg, themaximum effective dose; intraperitoneally) and paclitaxel vehicle (5%ethanol–5% Cremophor EL–saline; intravenously) or paclitaxel (20 mg/kg; the maximumtolerated dose in the A549 xenograft model; intravenously) were administered ondays 1, 8 and 15. On day 22, mice treated with bevacizumab plus paclitaxel werere-randomized into control and bevacizumab maintenance groups. Atrerandomization of the A549 xenograft mice, four mice were excluded to reducethe variability in tumor volume and body weight. As maintenance treatment,HuIgG or bevacizumab was administered weekly until day 78 in the A549 xenograftmodel and until day 50 in the NCI-H292 xenograft model. Tumor volume (TV) was measuredtwice a week. The antitumor activity was evaluated by TV on day 85 (A549 model)or day 57 (NCI-H292 model).
As the induction treatment in the NCI-H2228xenograft model, HuIgG or bevacizumab (5 mg/kg; maximum effective dose) and vehicle(saline) or pemetrexed (400 mg/kg; maximum effective dose) were administeredintraperitoneally on days 1, 8 and 15. On day 22, the mice treated withbevacizumab plus pemetrexed were rerandomized into four groups (control,bevacizumab, pemetrexed and bevacizumab plus pemetrexed). As the maintenancetreatment, HuIgG or bevacizumab and vehicle or pemetrexed were administered intraperitoneallyevery week until day 78. TV was measured twice a week. The antitumor activitywas evaluated by TV on day 85. TV was estimated from the equation TV=ab2/2,where a and b were tumor length and width, respectively.
Quantificationof microvessel density in tumor tissues
Microvessel density (MVD) in tumor tissueswas evaluated by immunohistochemical staining of cluster of differentiation 31(CD31). Tumor samples were collected on indicated days. Immunohistochemical stainingwas conducted. MVD was calculated as the percentage ratio of the CD31-positivestaining area to the total observation area in the viable region. Three to sixfields per section were analyzed, excluding necrotic areas. Positively stainedareas were calculated using imaging analysis software.
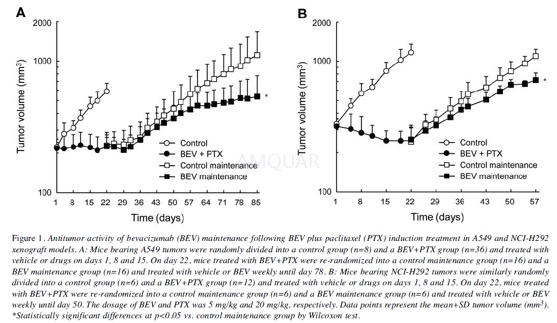

-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Ramezani, S.; Vousooghi, N.; Kapourchali, F. R.; Hadjighasem, M.; Hayat, P.; Amini, N.; Joghataei, M. T., Rolipram potentiates bevacizumab-induced cell death in human glioblastoma stem-like cells. Life Sci 2017, 173, 11-19.
[2] Ishikura, N.; Yanagisawa, M.; Noguchi-Sasaki, M.; Iwai, T.; Yorozu, K.; Kurasawa, M.; Sugimoto, M.; Yamamoto, K., Importance of Bevacizumab Maintenance Following Combination Chemotherapy in Human Non-small Cell Lung Cancer Xenograft Models. Anticancer Res 2017, 37 (2), 623-629.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们




















