-
生物活性
Avelumabbinds to both human and mouse PD-L1 with high affinity, Kd=0.3 and 1.0 nmol/L,respectively.[1]
-
体外研究
-
体内研究
-
激酶实验
Antibody competition assay[2]
PBMC from a cancer patient with mCRPC wererested for48 h and then pre-incubated for 30 min with avelumab or IgG1 isotype control at 0.2, 2, and 20 μg/mL, washed and then stainedwith multiparametric flow cytometry, to detect PD-L1 (with the MIH-1 clone) withinthe various immune cell types. PBMC that were not pre-incubated with avelumabor the isotype control also served as controls. The frequency of PD-L1 within eachof the classic immune cell types was determined both as a percentage of parentand total PBMC.
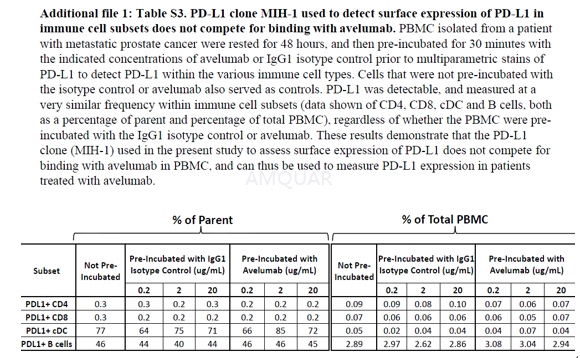
-
细胞实验
Cell lines and cultures[3]
The parental cell line NK-92 was originallyestablished from a male 50-year-old patient with non-Hodgkin lymphoma, whosebone marrow was infiltrated with large granular lymphocytes (LGL). The NK-92and a clone of NK-92 designated aNK are dependent on IL-2 for proliferation.NK-92 cells were genetically modified to produce IL-2 in an autocrine loop, aswell as to express a high affinity variant (V158) of the CD16 FcγRIII,and have been designated haNK cells. haNK cells are cultured in phenol freeXVivo-10 medium supplemented with 5% human heat-inactivated AB serum at aconcentration of 5x 105/ml. Human tumor cell lines (H441: lungcarcinoma; SKOV3: ovarian carcinoma; H460: lung carcinoma; HCC4006: lungcarcinoma; SW403: colorectal carcinoma; MDA-MB-231: breast carcinoma; andHTB-4: bladder carcinoma) were cultured freely of mycoplasma and maintained inRPMI-1640 supplemented with 10% FCS, 100 U/ml penicillin, 100μg/mlstreptomycin, and 2 mM L-glutamine.
Evaluationof haNK cell cytokine production
Culture supernatants were evaluated forsecretion of IFN-γ, IL-10, IL-2 and IL-8 using a multiplex cytokine/chemokine kit.
ADCCassay and blocking experiments
Irradiated (10 Gy) haNK cells, irradiated(10 Gy) aNK cells, and NK cells isolated from healthy donors were all used as effectorsin 111In-release lysis assays performed for 4 hr and/ or 18 hr. NKcells from healthy donors were isolated using the Human NK Cell Isolation(negative selection, Kit 130–092–657) following the manufacturer’sinstructions, resulting in>90% purity, or the EasySep NK cell isolation kitper the manufacturer’s instructions, resulting in >90% purity. Prior toassay, cells were rested overnight in RPMI-1640 medium containing 10% human AB serum.Target cells were labeled with 20 μCi of 111In-oxyquinolineat 37oC for 20 min and used as targets at 2–3,000 cells/ well in a96-well round-bottom culture plate at various effector to target cell (E:T)ratios as indicated.
For ADCC experiments, the targets werefirst incubated for 30 min with the test MAb and isotype control MAb before thehaNK cells were added. The plates were incubated for 4 hr or 18 hr at 37oCin a humidified atmosphere containing 5% CO2, then harvested andcounted on a Wizard2 gamma counter. All samples were tested intriplicate and specific lysis was calculated from the average. Spontaneousrelease was determined by incubating targets with medium alone; complete lysiswas determined by incubating targets with 0.05% Triton X-100. Specific lysis wasdetermined using the following equation: Percent lysis= (experimental – spontaneous)/(complete– spontaneous) x 100.
For the blocking experiments, irradiatedhaNK cells were preincubated for 2 hr with either anti-CD16 antibody (50 μg/ml,clone B73.1) or concanamycin A (CMA; 200 nM) before being used in lysis assayswith the H460 human lung carcinoma cell line as a target at a 25:1 E:T ratio.Both NK lysis and ADCC assays mediated by avelumab or cetuximab were performedin 4-hr or 18-hr 111In release assays as indicated.
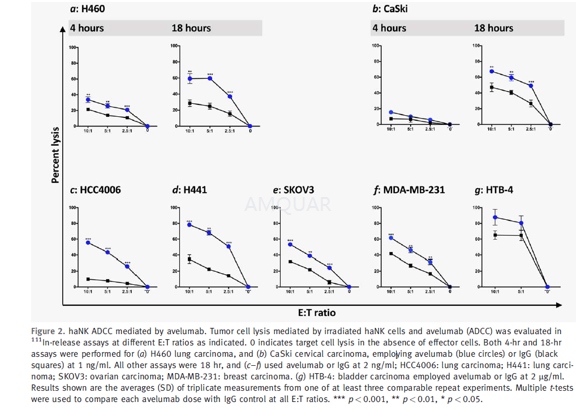
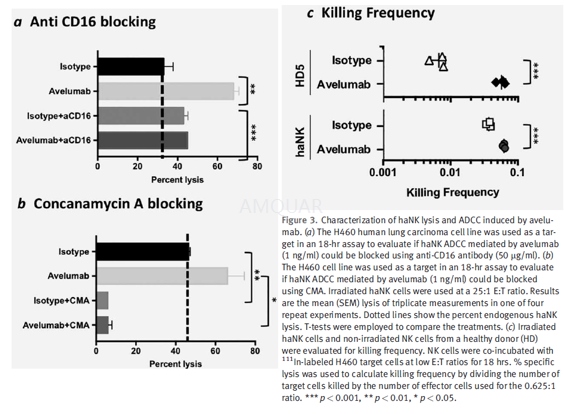
-
动物实验
Animals[1]
Female C57BL/6 mice were housed in microisolatorcages in pathogen-free conditions. Mice used for the in vivo antitumor studieswere 16 to 18 weeks old at the start of study.
Murinetumor models
Subcutaneous tumor injections were carriedout by inoculating C57BL/6 mice with 1 x 105 MB49 parental cells onthe right shaved flank. Tumor growth was measured with calipers, and 8 daysafter inoculation, mice were assigned to treatment groups. Treatments began onday 9, tumors were measured 2x/week throughout the study, and tumor volume wascalculated as: Volume = (width)2 x (length)/2. Mice were euthanizedwhen the tumor size was greater than 1.5 cm3.
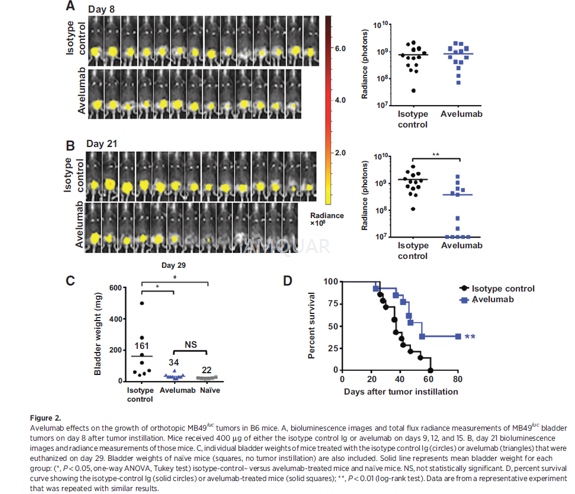
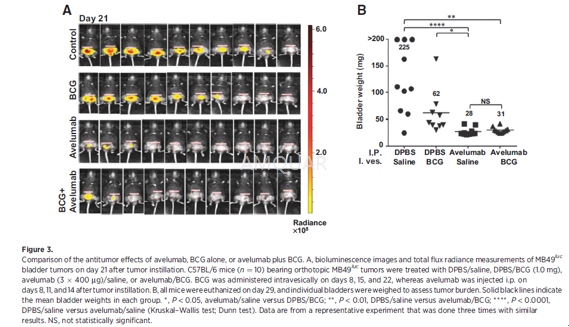
Intravesical instillation of orthotopicMB49luc bladder tumors was carried out. Mice were anesthetized [ketamine(15 mg/kg) and xylazine (75 mg/kg)] and catheterized using Teflon catheters(SurFlash Polyurethane I.V. catheter 24G x 3/4, SR *FF2419). Orthotopic bladdertumors were established by instilling 7.5 x 104 MB49luc cellsfor 45minutes into bladders that had been pretreated with 100 μL of poly-L-lysine (0.1μg/mL) solution [PLL, molecularweight (MW) 70,000 to 150,000]. Tumor take was confirmed by in vivo imaging 7to 10 days later, at which time mice were placed into groups with equal tumorburden prior to treatment.
Immunecell subset depletion
Immune cell subset depletions were carriedout by administering anti-CD4 (100μg GK1.5) or anti-CD8 (100μg2.43) T-cell–depletion antibodies each day for 4 consecutive days andthereafter with weekly injections. Natural killer (NK)-cell depletion was achievedby weekly administration of 25 mL rabbit anti–asialo-GM1. The administration ofthe depleting antibodies was begun 1 week prior to the intravesical tumor cell instillation.Depletions were confirmed with flow cytometric analyses for CD4 and CD8 cellsand NK lysis of YAC-1 targets using splenocytes from representative mice
Treatments
Avelumab and an isotype-control antibodywere diluted in Dulbecco's phosphate buffered saline (DPBS) prior to injection.Tumor-bearing mice were treated with 400μg per 100μLand injected i.p. three times, 3 days apart. Because avelumab is a human IgG1,three injections had to be compressed within a 7- to 9-day window (i.e., days9, 12, and 15 after tumor inoculation) to avoid the onset of neutralizing mouseanti-human Ig.
BCG (1.0 mg) was administered in a volumeof 100μL instilled by catheterization for 1 hour, three times at weeklyintervals starting on day 8 after tumor instillation.
Bioluminescentimaging
Orthotopic bladder tumor growth wasdetected by IVIS Lumina In-vivo Imaging. The abdominal regions of the mice wereshaved, and the mice were injected i.p. with luciferin salt (15 mg/kg), 10minutes before imaging. Temporary anesthesia was administered by Forane(Isoflurane) inhalation at 2% for the duration of imaging. The acquiredbioluminescence signals in a region of interest over the tumor site were readout as total flux radiance [photons/sec/cm2/steradian (sr)] usingthe Living Image software, Version 4.1
Histologyand immunohistochemistry
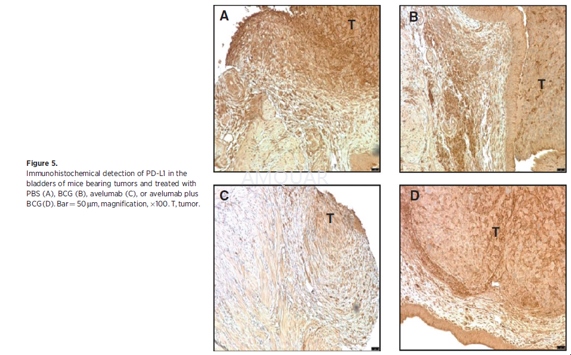
For pathologic evaluation, tissues (i.e.,bladder, lungs, liver, kidneys, etc.) were removed for gross examination.Bladder tissue was fixed in 10% neutral buffered formalin, embedded in paraffinblocks, and processed by routine histologic methods for hematoxylin and eosin(H&E) staining. The remaining bladder tissue was embedded in optimalcutting temperature (OCT) compound for staining of CD3 (clone OKT3),CD4(cloneRM4-5), CD8a (clone 53-6.7), and PD-L1 (clone AF1019;) as well as H&E. Ithas been reported that instillation of a higher number of MB49luc tumor cells (i.e., 106) into mouse bladders previously traumatizedwith silver nitrate resulted in lung metastases. Upon examination of theH&E-stained slides, a board-certified pathologist found the urinary bladderpathology to be "carcinoma, transitional cell, malignant withoutmetastasis" with no accompanying metastatic lesions in the lungs. Stainedslides were scanned using the Leica Biosystems anatomic pathology scanner. TheAperio Image Scope analyses were completed using positive pixel count, which determinedthe total number of positive pixels (Ntotal-Nnegative= TNP)for each corresponding graph. TNP values were compared on an individualtreatment group basis and analyzed with respect to region (bladder/tumorinterface, tumor) and size (cells/micron squared; i.e., TNP cells/mm2).The bladder/tumor interface was defined as 50 mm in either direction from thebladder/tumor border. Higher magnification images were taken on a Leica DMI4000microscope with LAS AF.
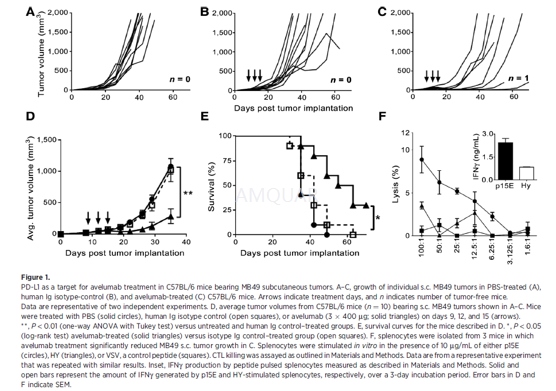
-
不同实验动物依据体表面积的等效剂量转换表(数据来源于FDA指南)
|  动物 A (mg/kg) = 动物 B (mg/kg)×动物 B的Km系数/动物 A的Km系数 |
|
例如,已知某工具药用于小鼠的剂量为88 mg/kg , 则用于大鼠的剂量换算方法:将88 mg/kg 乘以小鼠的Km系数(3),再除以大鼠的Km系数(6),得到该药物用于大鼠的等效剂量44 mg/kg。
-
参考文献
[1] Vandeveer, A. J.; Fallon, J. K.; Tighe, R.; Sabzevari, H.; Schlom, J.; Greiner, J. W., Systemic Immunotherapy of Non-Muscle Invasive Mouse Bladder Cancer with Avelumab, an Anti-PD-L1 Immune Checkpoint Inhibitor. Cancer Immunol Res 2016, 4 (5), 452-62.
[2] Donahue, R. N.; Lepone, L. M.; Grenga, I.; Jochems, C.; Fantini, M.; Madan, R. A.; Heery, C. R.; Gulley, J. L.; Schlom, J., Analyses of the peripheral immunome following multiple administrations of avelumab, a human IgG1 anti-PD-L1 monoclonal antibody. J Immunother Cancer 2017, 5, 20.
[3] Jochems, C.; Hodge, J. W.; Fantini, M.; Tsang, K. Y.; Vandeveer, A. J.; Gulley, J. L.; Schlom, J., ADCC employing an NK cell line (haNK) expressing the high affinity CD16 allele with avelumab, an anti-PD-L1 antibody. International Journal of Cancer 2017, 141 (3), 583-593.
分子式
|
分子量
|
CAS号
|
储存方式
-80 ℃长期储存。干冰运输 |
溶剂(常温)
|
DMSO
|
Water
|
Ethanol
|
体内溶解度
-
Clinical Trial Information ( data from http://clinicaltrials.gov )
注:以上所有数据均来自公开文献,并不保证对所有实验均有效,数据仅供参考。
-
相关化合物库
-
使用AMQUAR产品发表文献后请联系我们























